

Physical & Chemical Properties of Water

Core Concepts
In this tutorial on the properties of water, you will learn about the physical and chemical properties of water. You will also learn about the structure of a water molecule.
Topics Covered in Other Articles
Polarity of Water
Electronegativity
Solvent v.s. Solute
Specific Heat
K w of Water
Density- Mass per unit volume
Specific Heat Capacity- Amount of energy required to raise the temperature of 1 kg of a material by 1 C
Heat of Vaporization- Amount of energy required to transform a quantity of a liquid into a gas
Polar Molecule- A molecule with a partially negative charged end and a partially positive charged end
Electronegativity- Tendency for an atom to attract shared electrons in a chemical bond
Introduction to Properties of Water
Water (H20) is the “universal solvent” and the most abundant surface on Earth. It is also the only common substance to exist as a solid, liquid, and gas naturally. Water molecules form hydrogen bonds and are extremely polar. The five main properties of water are its high polarity, high specific heat, high heat of vaporization, low density as a solid, and attraction to other polar molecules.
Polarity and Structure
One oxygen atom and two hydrogen atoms make a water molecule. It has a bent molecular geometry with the oxygen having two lone pairs of electrons. The difference in electronegativity between the oxygen and hydrogens causes the oxygen to have a partial negative charge and the hydrogen to have a partial positive charge. This difference in charge causes polarity. The partial positive charge of the hydrogen of one water molecule attracts the partial negative charge of the oxygen of another water molecule. This attraction is called hydrogen bonding.
Hydrogen bonding is weaker than the covalent bonds between the oxygen and hydrogens of the same molecule but causes many of water’s unique properties. For example, more energy is required to break hydrogen bonds, so water has a higher melting and boiling point .
Universal Solvent
Water is the solvent of life. Hydrophilic substances are those that dissolve in water, while hydrophobic substances do not mix well with water. Substances can dissolve in water if they can match or overcome the hydrogen bonding between water molecules. If they cannot, the substance forms a precipitate. Acids , alcohols, and salts are rather soluble in water while fats and oils are hydrophobic.
An ionic solute dissolved in water becomes separated. For example, NaCl separates into Na+ cations and Cl- anions surrounded by water molecules. Water is amphoteric, meaning it can act as either an acid or a base depending on the solution. It can produce both H+ and OH- ions.
Specific Heat Capacity
Water has a high specific heat of 4184J/(kg x K) at 20 C and high heat of vaporization because of hydrogen bonding. This allows bodies of water to have minimal fluctuations in temperature to regulate climate.
Water’s high heat of vaporization allows humans to use sweat to cool off. Sweat is mostly made of water. It absorbs excess body heat as it evaporates. This process is known as evaporative cooling.
Water’s density is 1 gram per cubic cm. This is used to define the gram. Instead of undergoing thermal expansion, the density rises with temperature to a peak of 3.98 C and then decreases. Negative thermal expansion is the increase in density between 32 and 39.16 F. As a result, ice is less dense than water, which has a decrease in density by about 10%. This is why bodies of water may have a layer of ice on the surface but contain liquid underneath. This allows fish and marine life to survive under the ice. High specific heat keeps the temperature of the water relatively stable through the winter so that marine life can survive.
Salt content lowers the freezing point of the ocean by almost 2 C. Ice still floats on the ocean, but the ice is near salt-free with a similar density to ice on bodies of freshwater. The salt adds to the salinity and density of the remaining water which sinks by convection. This process is called brine rejection.
Compressibility
Compressibility is a result of pressure and temperature. The compressibility of water is so low that is often assumed to be incompressible. Low compressibility allows water in deep oceans with high pressure to only decrease by 1.8% in volume.
Electrical Conductivity
Pure water is a good insulator, but deionized water is never completely free of ions. Water undergoes a process called autoionization as a liquid. This means that two water molecules can form one hydroxide anion (OH-) and one hydronium cation (H 3 0+).

Cohesion and Adhesion
Hydrogen bonds between water molecules are constantly breaking and reforming with other water molecules. Cohesion is the ability of water molecules to stick together. Water’s polarity also gives water high adhesion: the ability to stick to other surfaces. The adhesive forces are stronger than cohesive forces.
Strong cohesion and adhesion cause water to exhibit capillary action. Capillary action is the process of liquid flowing through a narrow space without and often against gravity. Water adheres to the walls of plants’ roots and rises up into the plant. Porous materials such as water also exhibit capillary action. Trees can transport water through capillary action over 100 meters.
Surface Tension
The hydrogen bonding also causes water to have a high surface tension of 71.99mN/m at 25. The surface tension is high enough for insects to walk on water. Surface tension is a result of water’s cohesive properties. Water droplets and water rising above the rim of a glass show water’s high surface tension. Learn more about the properties of water here.
Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
Chapter 3 Chemistry and Life
3.2 The Chemical and Physical Properties of Water
By the end of this section, you will be able to:
Describe the properties of water that are critical to maintaining life
Distinguish between hydrophilic and hydrophobic molecules
Explain why water is a good solvent for many solutes
Explain the pH scale using the specified terms; indicate how much more (or less) acidic one substance is compared to another using the pH scale
Do you ever wonder why scientists spend time looking for water on other planets? It is because water is essential to life; even minute traces of it on another planet can indicate that life could or did exist on that planet. Water is one of the more abundant molecules in living cells and the one most critical to life as we know it. Approximately 60–70 percent of your body is made up of water. Without it, life simply would not exist.
Water Is Polar
The hydrogen and oxygen atoms within water molecules form polar covalent bonds. The shared electrons spend more time associated with the oxygen atom than they do with hydrogen atoms. There is no overall charge to a water molecule, but there is a slight positive charge on each hydrogen atom and a slight negative charge on the oxygen atom. Because of these charges, the slightly positive hydrogen atoms repel each other. Each water molecule attracts other water molecules because of the positive and negative charges in the different parts of the molecule. Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. When a substance readily forms hydrogen bonds with water, it can dissolve in water and is referred to as hydrophilic (“water-loving”). Hydrogen bonds are not readily formed with nonpolar substances like oils and fats. These nonpolar compounds are hydrophobic (“water-fearing”) and will not dissolve in water (Figure 2.8 ).
Water Stabilizes Temperature
The hydrogen bonds in water allow it to absorb and release heat energy more slowly than many other substances. Temperature is a measure of the motion (kinetic energy) of molecules. As the motion increases, energy is higher and thus temperature is higher. Water absorbs a great deal of energy before its temperature rises. Increased energy disrupts the hydrogen bonds between water molecules. Because these bonds can be created and disrupted rapidly, water absorbs an increase in energy and temperature changes only minimally. This means that water moderates

temperature changes within organisms and in their environments. As energy input continues, the balance between hydrogen-bond formation and destruction swings toward the destruction side. More bonds are broken than are formed. This process results in the release of individual water molecules at the surface of the liquid (such as a body of water, the leaves of a plant, or the skin of an organism) in a process called evaporation . Evaporation of sweat, which is 90 percent water, allows for cooling of an organism, because breaking hydrogen bonds requires an input of energy and takes heat away from the body.
Water Is an Excellent Solvent
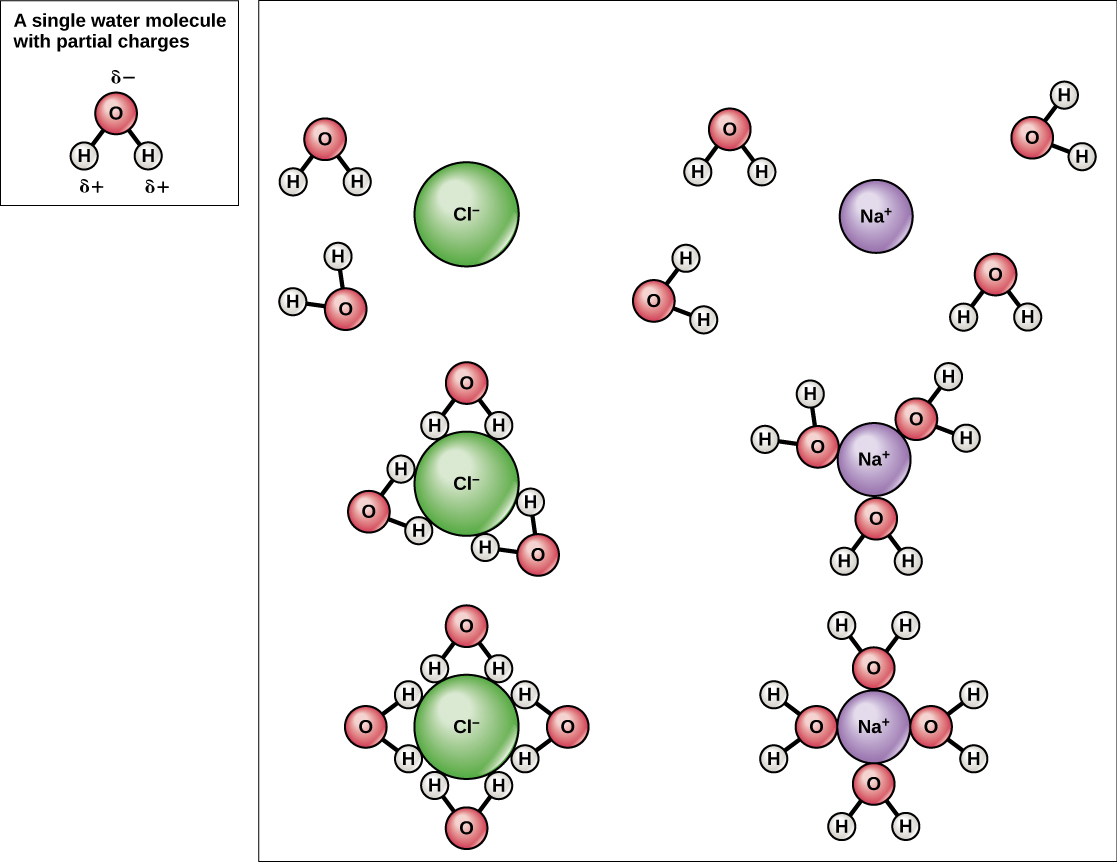
Because water is polar, with slight positive and negative charges, ionic compounds and polar molecules can readily dissolve in it. Water is, therefore, what is referred to as a solvent —a substance capable of dissolving another substance, referred to as the solute, in order to form a solution. The charged particles will form hydrogen bonds with a surrounding layer of water molecules. This is referred to as a sphere of hydration and serves to keep the particles separated or dispersed in the water. In the case of table salt (NaCl) mixed in water (Figure 2.9 ), the sodium and chloride ions separate, or dissociate, in the water, and spheres of hydration are formed around the ions. A positively charged sodium ion is surrounded by the partially negative charges of oxygen atoms in water molecules. A negatively charged chloride ion is surrounded by the partially positive charges of hydrogen atoms in water molecules. The polarity of the water molecule makes it an effective solvent and is important in its many roles in living systems.
Buffers, pH, Acids, and Bases
The pH of a solution is a measure of its acidity or alkalinity. You have probably used litmus paper , paper that has been treated with a natural water-soluble dye so it can be used as a pH indicator, to test how much acid or base (alkalinity) exists in a solution. You might have even used some to make sure the water in an outdoor swimming pool is properly treated. In both cases, this pH test measures the amount of hydrogen ions that exists in a given solution. High concentrations of hydrogen ions yield a low pH, whereas low levels of hydrogen ions result in a high pH. The overall concentration of hydrogen ions is inversely related to its pH and can be measured on the pH scale (Figure 2.10 ). Therefore, the more hydrogen ions present, the lower the pH; conversely, the fewer hydrogen ions, the higher the pH.
The pH scale ranges from 0 to 14. A change of one unit on the pH scale represents a change in the concentration of hydrogen ions by a factor of 10, a change in two units represents a change in the concentration of hydrogen ions by a factor of 100. Thus, small changes in pH represent large changes in the concentrations of hydrogen ions. Pure water is neutral. It is neither acidic nor basic, and has a pH of 7.0. Anything below 7.0 (ranging from 0.0 to 6.9) is acidic, and anything above 7.0 (from 7.1 to 14.0) is alkaline. The blood in your veins is slightly alkaline (pH = 7.4). The environment in your stomach is highly acidic (pH = 1 to 2). Orange juice is mildly acidic (pH = approximately 3.5), whereas baking soda is basic (pH = 9.0) (Figure 2.10 ).

Acids are substances that provide hydrogen ions (H + ) and lower pH, whereas bases provide hydroxide ions (OH − ) and raise pH. The stronger the acid, the more readily it donates H + . For example, hydrochloric acid and lemon juice are very acidic and readily give up H + when added to water. Conversely, bases are those substances that readily donate OH − . The OH − ions combine with H + to produce water, which raises a substance’s pH. Sodium hydroxide and many household cleaners are very alkaline and give up OH − rapidly when placed in water, thereby raising the pH.
Most cells in our bodies operate within a very narrow window of the pH scale, typically ranging only from 7.2 to 7.6. If the pH of the body is outside of this range, the respiratory system malfunctions, as do other organs in the body. Cells no longer function properly, and proteins will break down. Deviation outside of the pH range can induce coma or even cause death.
So how is it that we can ingest or inhale acidic or basic substances and not die? Buffers are the key. Buffers readily absorb excess H + or OH − , keeping the pH of the body carefully maintained in the aforementioned narrow range. Carbon dioxide is part of a prominent buffer system in the human body; it keeps the pH within the proper range. This buffer system involves carbonic acid (H 2 CO 3 ) and bicarbonate (HCO 3 − ) anion. If too much H + enters the body, bicarbonate will combine with the H + to create carbonic acid and limit the decrease in pH. Likewise, if too much OH − is introduced into the system, carbonic acid will rapidly dissociate into bicarbonate and H + ions. The H + ions can combine with the OH − ions, limiting the increase in pH. While carbonic acid is an important product in this reaction, its presence is fleeting because the carbonic acid is released from the body as carbon dioxide gas each time we breathe. Without this buffer system, the pH in our bodies would fluctuate too much and we would fail to survive.
Section Summary
Water has many properties that are critical to maintaining life. It is polar, allowing for the formation of hydrogen bonds, which allow ions and other polar molecules to dissolve in water. Therefore, water is an excellent solvent. The hydrogen bonds between water molecules give water the ability to hold heat better than many other substances. As the temperature rises, the hydrogen bonds between water continually break and reform, allowing for the overall temperature to remain stable, although increased energy is added to the system. All of these unique properties of water are important in the chemistry of living organisms.
The pH of a solution is a measure of the concentration of hydrogen ions in the solution. A solution with a high number of hydrogen ions is acidic and has a low pH value. A solution with a high number of hydroxide ions is basic and has a high pH value. The pH scale ranges from 0 to 14, with a pH of 7 being neutral. Buffers are solutions that moderate pH changes when an acid or base is added to the buffer system. Buffers are important in biological systems because of their ability to maintain constant pH conditions.
Review Questions
Introduction to Human Biology Copyright © by Wolf T Pecher is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License , except where otherwise noted.
Share This Book

IMAGES
VIDEO