April 28, 2016

React Fast: How Size Determines Rate
A fizzy science project from Science Buddies
By Science Buddies
Bubble up with this fun test of reaction times. See which size makes the biggest fizz!
George Retseck
Key concepts Chemistry Physics Reaction Surface area
Introduction Did you know that flour can explode? Luckily, this does not happen spontaneously on your kitchen counter, but only if the conditions are right. You need a very fine powder of flour to make an explosion happen. In fact, any solid flammable material that is dispersed in the air as a dust cloud will explode if it comes into contact with flame (a reason extreme caution must be used where there is a large amount of grain dust, such as in storage facilities). Why is that? It has to do with the particle size of the solid material, which determines how rapidly a chemical reaction takes place. In this activity, you can try this for yourself—skipping the explosion and creating a big fizz instead!
Background Some chemical reactions happen very fast (think vinegar and baking soda), whereas others take a very long time (such as rust forming on metal). In chemical reactions that include a solid as one of the reactants, you can actually change the reaction rate by varying the size of the solid that reacts with the liquid or the gas. How does this work? For a chemical reaction to happen, the molecules or atoms of the reactants need to collide with each other. This can only happen at the surface of the solid, as all the molecules trapped within the body of the solid cannot react until they meet the molecules of the other reactant. However, if you take the same material and break it into smaller pieces, there is much more surface area exposed that can interact with the other components—allowing the chemical reaction to occur much more quickly.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Take a flammable material (such as flour) as an example: If you heat up a lump of flour, it will not burn at all or will start burning away slowly in a controlled manner because there is only a limited surface area available that can react with the oxygen in the air. However, the same flammable material dispersed into the air as a fine powder would allow for much greater surface area exposed to the air, allowing for a rapid explosion if ignited. Increasing the surface area of a reactant does not only increase the quantity of material available to react, but will also increase the rate of the reaction. In this activity, you will demonstrate this effect by measuring the rate of a different kind of chemical reaction: the dissolution of sodium bicarbonate from an effervescent antacid tablet in water.
Effervescent antacid tablets (at least four)
Sheet of paper
Four clear 12-ounce (or larger) drinking glasses
Measuring cup
Preparation
Take four antacid tablets out of their packages. Take care not to break them, as the tablets are very brittle.
Put one whole antacid tablet aside for now.
Take the second tablet and break it into half and set both halves aside.
Take the third tablet and break it evenly into quarters. First, break it into halves and then break the halves again into two parts. Set all four pieces aside.
Take the fourth tablet and ground it into a powder. To do this, put the tablet to be ground inside a clean, folded piece of paper. Place the folded paper on a solid surface and use a spoon to carefully crush the tablet into a powder. Keep the powder folded into the paper and set it aside.
Use a measuring cup to add about 250 milliliters (about eight ounces) of tap water to each of the four glasses. The temperature of the water should be the same in each.
Take one of the glasses with tap water and the whole antacid tablet and put them in front of you.
Pick up the whole tablet and hold it above the water surface.
Get your stopwatch ready.
Drop the whole tablet into the water and at the same time start the stopwatch. What happens once the tablet hits the water? Can you see a chemical reaction happening?
Stir the water gently and steadily with the teaspoon. Observe the tablet closely in the water. What do you notice about the tablet? What reaction do you think is taking place?
Once all the solid material of the tablet has dissolved in the water and the chemical reaction is completed, stop the stopwatch and write down the reaction time on a sheet of paper. How long did the reaction take? Do you think this reaction is fast or slow?
Get a fresh glass of water and this time take the antacid tablet that you broke in half.
Take both pieces of the tablet and hold them above the water surface. What do you think will change once you put the two pieces of the tablet into the water compared to the whole tablet?
Reset your stopwatch and get it ready.
Drop both pieces of the tablet into the water and start the timer again. Compared to the whole tablet, do you see the same reaction happening in the water?
Again, stir the water gently and observe how the two tablet pieces dissolve in the water. Do you see more or fewer bubbles forming? Do you think this reaction will be complete faster or more slowly than with the whole tablet?
Once all the solid tablet material has completely disappeared and the bubbles have stopped forming, stop the stopwatch and record the reaction time. Did the reaction time change compared to the whole tablet? Was this reaction faster or slower? Why do you think this is the case?
With the two remaining glasses, repeat the antacid-adding steps with the antacid tablet that you broke into four pieces and the tablet that you crushed into a powder. Do you observe any changes in the chemical reaction happening in the water? How fast or slow are these tablets dissolving compared to the other tablets? Do you notice any correlation between the reaction time and the size of the tablet pieces?
Extra: Can you think of other chemical reactions that you could use to test how the surface area of one of the reactants affects the reaction rate with water? Think of other ingredients in your kitchen that come in various sizes and forms, such as sugar crystals, cubes or powder. Will the same effect be observable for these substances?
Extra: What other factors can change the rate of a chemical reaction? Repeat this activity, but only use whole antacid tablets, and this time, vary the temperature of the water in which you dissolve the tablets. How do you think the temperature will influence the reaction rate? Will the tablet dissolve faster or more slowly in hot water compared to cold?
Observations and results Did you find that the tablet powder dissolved much faster than the whole tablet? What you probably observed in all of your trials was some vigorous bubbling once you dropped the antacid tablet into the water. Effervescent antacid tablets are made from aspirin, citric acid and sodium bicarbonate. When sodium bicarbonate dissolves in water, it reacts with hydrogen ions from the citric acid and forms carbon dioxide. Because carbon dioxide is a gas, it forms bubbles inside the water that you can see as foam on the surface.
The fizzing and bubbling was probably more pronounced the smaller the tablet pieces were that you dropped into the water. At the same time, you probably noticed that the whole tablet took the longest to dissolve, whereas the tablet powder dissolved really quickly. This is because with smaller tablet pieces, there is more surface area of the tablet available that can react with the water, which results in a faster disintegration of the antacid tablet, as you observed.
Cleanup Pour the water with the dissolved antacid tablets into a sink.
More to explore Surface Area and Reaction Rate , from ADLC Educational Media Harmless Flour Is an Incredibly Explosive Substance , from AweSci Big Pieces or Small Pieces: Which Reacts Faster? , from Science Buddies Science Activity for All Ages!, from Science Buddies
This activity brought to you in partnership with Science Buddies

Middle School Chemical Engineering For Girls
Great Activities for Middle School Outreach in Chemical Engineering

The Alka Seltzer Reaction
Introduction & motivation.
Chemical reactions are one of the primary focuses for Chemical Engineers. From synthesizing polymers to treating water to creating fertilizers, chemical reactions are important in nearly every aspect of daily life. One job of Chemical Engineers is to classify, understand, and control these reactions to speed them up or slow them down.
Chemical reactions occur when bonds within molecules are broken or formed. There are several things that signify that a chemical reaction took place. These include a change in color, the production of a gas or solid, and of course a change in chemical composition. The starting chemicals before a reaction are called the reactants , and the chemicals that are produced are called the products . The reaction in this activity involves using sodium bicarbonate and citric acid to produce water and carbon dioxide.
Reaction : HCO 3 – (aq) + H + (aq) → H 2 O (l) + CO 2 (g)
The tablets contain sodium bicarbonate (NaHCO 3 ) and citric acid. When the tablet is dissolved in water, bicarbonate (HCO 3 – ) and hydrogen ions (H + ) are formed. Once in solution, the two chemicals can then react according to the reaction listed above. For the reaction to occur, the HCO 3 – and H + must collide at the right angle with the right amount of energy. The chances of this happening are better when the tablet is crushed into more pieces since the molecules have more opportunities to collide and when the temperature is higher, since the molecules are moving faster.
In this activity, students will experiment with the reaction between Alka Seltzer tablets and water in different conditions. By changing temperature and the surface area available for reaction, students will begin to see what factors chemical engineers can control to get the desired result.
This activity introduces the reaction used for the Alka Seltzer Rockets activity, so it is typically performed before building rockets to understand the nature of the reaction before using it.
Chemical Safety:
- Sodium Bicarbonate
- Alka Seltzer tablets
- Large beakers
- Food coloring
- Stopwatches
- Metal spoons
- Thermometers
Before the experiment, ask students to hypothesize what will make the reaction go the fastest and what makes them think that. This can be anything, but try to seek answers with specific regard to the variables being changed in this activity.
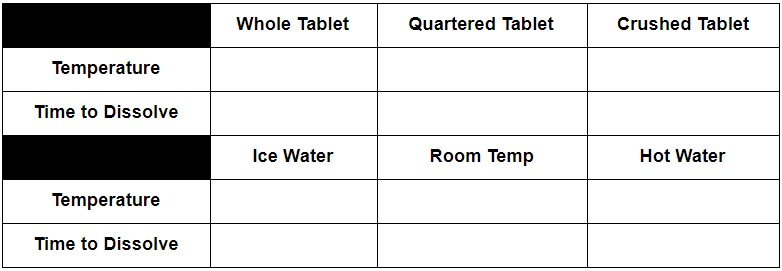
The Effect of Temperature on Rate of Reaction
- Partially fill a large beaker with ice cubes. Fill the beaker with water up to the 250 mL mark with cold water and stir the ice water until the temperature equilibrates.
- Measure the temperature of the water and record it in the table.
- Add a tablet and record the time it takes for the tablet to react.
- Repeat 1-2 with room temperature water, then with hot water heated to 70 degrees C using a hot plate.
The Effect of Surface Area on Rate of Reaction
- A whole tablet
- A tablet broken into quarters
- A tablet ground into powder: Place the tablet it a piece of weighing paper (wax or parchment paper work as well) and break it either with your hands or crush it using the back of a metal spoon.
- Add 250 mL of water to a large beaker.
- Measure and record the temperature of the water and make sure it is consistent between trials.
- One student should be ready with a stopwatch and another student should be ready with the whole tablet. The student with the stopwatch should count to three and on three start the stopwatch. At the same time, the other student should drop the tablet into the water.
- Gently stir the water at a consistent speed and pattern.
- As soon as the last of the tablet disappears, yell “Stop!,” stop the stopwatch, and record the time in the table.
- Repeat Steps 2-6 with the quartered tablet and the crushed tablet.
At the end, collect and present all class data on the board. Highlight discrepancies and the general trend.
- Which combination of factors made the reaction go the fastest? The slowest? (Higher surface area and temperature make the reaction go faster. Since the reaction occurs on the surface of the tablet pieces, more access to it will make the reaction go faster because there are more molecules to make bumping together more likely. Higher temperature gives more energy to the molecules, meaning they are more likely to have enough energy for the reaction to continue. The opposite is true for the slowest rate – low surface area and temperature.)
- Why would we want reactions to happen faster or slower? (e.g. we want rusting reactions to be slower to protect metal products, but we want redox reactions that recharge our phone batteries to be fast.)
- Is there a limit to how fast we can make the reaction? Would we want to place a limit if there is not a physical one? (Reactions have maximum rates for a few reasons, like the amount of surface area available to react, if the mixture makes it difficult for molecules to move, etc. If the rate were increased too high, it becomes a safety concern! Sometimes reactions get too fast, too hot, and can’t be slowed down. This is a dangerous runaway reaction , the last thing a chemical engineer wants!)
- Why did any discrepancies come up in the data? What ways could we make our process better to limit those from affecting the class data as a whole? (Discrepancies come up from human error with measuring time, not having precise sizes of tablets, imprecise temperature control across trials, and how hard it is to see a reaction is finished! Let students get creative with suggesting improvements, but a few could include using a grid and knives to chop up tablets or putting the ground tablets through a sieve, using a robot to stir and observe the reaction, and putting the beakers in water baths.)
- We know Alka Seltzer is a medicine to make us feel better. Why might it be designed to fizz? (Fizzing helps the aspirin in the tablet quickly absorb into the bloodstream, making the medicine fast-acting. It might also make it more appetizing to drink!)
Additional Resources
- How Does Alka Seltzer Work?
- VIDEO: Why Does Alka Seltzer Fizz?
- ← Alka Seltzer Rockets
- Separations Activity →
Leave a Reply Cancel reply
You must be logged in to post a comment.
Disclaimer | Non-Discrimination | Privacy | Terms for Creating and Maintaining Sites

Surface Area

Explore how a material can act differently when it’s small than when it’s big. Test the reaction of fizzing antacid tablets in water and find out whether the size of the pieces makes a difference!
Age : 6+ Time : 15 minutes Topics : antacid, surface area, chemistry, reaction
What you need:
- 2 matching clear glasses (small and narrow works best)
- Masking tape
- Ruler or measuring tape
- 2 small measuring cups of the same size (¼ -½ cup works well), or 1 measuring cup and an extra cup or glass
- Pitcher or large cup for pouring
- Effervescent (fizzing) antacid tablets
- Water
- Food coloring (optional)
What to do:
1. Before you start this activity, do a little research. Put one antacid tablet on a plate or in a small bowl, drop or pour a tiny bit of water on it and make some observations about what happens. What did you see when you first put water on it? Did you hear anything? What happened to the tablet after a minute or two? Add a little bit more water, what happened next? Record your observations by taking a photo, drawing a picture, or writing some notes.
2. Turn your two matching glasses into measuring tools using the tape and ruler. Put a piece of tape lengthwise up the side of each glass, all the way from bottom to top. Use the ruler and marker to mark lines on the tape in one-inch increments starting from the bottom of the glass.
3. Fill the large cup or pitcher with water. If you like, add a few drops of food coloring to it to make the water easier to see.
4. Pour the water from the large cup or pitcher into each of the two measuring cups. Make sure both cups have the same amount of water in them. Tip: If you don’t have two measuring cups of the same size, fill the measuring cup once and empty it into an extra cup. Then fill the measuring cup again.
5. Take one antacid tablet and break it in half. Place both halves in one of the clear glasses. Break a second tablet into many small pieces and place that in the other clear glass.
6. Before you add the water, make a prediction about what you think will be different between the two glasses. Will one react more quickly? Will one have a bigger reaction?
7. Now, pick up the two measuring cups. At the same time, pour the water from each measuring cup into one of the glasses of antacid pieces.
8. Observe what happens in each glass. Check the measurement markings on the tape to see if they help you notice any differences. Record your observations as you did in Step 1.
- What did you notice? In what ways were the two reactions different?
- How did those observations compare to your predictions?
9. Other ideas to try:
- Try the experiment again, but carefully stir in a drop or two of liquid dish soap or hand soap to each measuring cup of water before adding it to the glasses. How does this change what you see?
- Try the experiment again with different sized antacid pieces, for example: a whole tablet, a tablet in four pieces, or crushed completely into a powder. Be sure to use the same amount of water each time. How do those sizes compare to the sizes in your first test?
- What other factors might affect the antacid tablet’s reaction? The amount of water, the temperature of the water, the shape of the container? Design an experiment to test one of those factors.
- Does surface area affect how other materials dissolve in water? Try comparing a whole sugar cube or piece of hard candy to one that is crushed into pieces. What other materials could you try?
What’s happening?
These antacid tablets fizz when they come in contact with water because of a chemical reaction . The water reacts with the tablet to create bubbles full of carbon dioxide gas. Chemistry has a lot to do with chemical reactions, but it also studies properties of materials. The property at work in this experiment is surface area . Small things have more surface area for their volume than larger things do. Some things that aren’t reactive at all in big pieces are very reactive when they’re tiny. The smaller the pieces of the tablet the faster it fizzes because the smaller pieces have more surface area. For the same amount of antacid, the crushed tablet has more surface, or exterior, to react with the water. Since the water can reach more of the tablet immediately, the reaction, or fizzing, happens faster.
- Buy Tickets
- TFI Transformation
- Accessibility
- Frequently Asked Questions
- Itineraries
- Daily Schedule
- Getting Here
- Where to Eat & Stay
- All Exhibits & Experiences
- The Art of the Brick
- Wondrous Space
- Science After Hours
- Heritage Days
- Franklin Fright
- Events Calendar
- Staff Scientists
- Benjamin Franklin Resources
- Scientific Journals of The Franklin Institute
- Professional Development
- The Current: Blog
- About Awards
- Ceremony & Dinner
- Sponsorship
- The Class of 2024
- Call for Nominations
- Committee on Science & The Arts
- Next Generation Science Standards
- Title I Schools
- Neuroscience & Society Curriculum
- STEM Scholars
- GSK Science in the Summer™
- Missions2Mars Program
- Children's Vaccine Education Program
- Franklin @ Home
- The Curious Cosmos with Derrick Pitts Podcast
- So Curious! Podcast
- A Practical Guide to the Cosmos
- Archives & Oddities
- Ingenious: The Evolution of Innovation
- The Road to 2050
- Science Stories
- Spark of Science
- That's B.S. (Bad Science)
- Group Visits
- Plan an Event
- Renew Membership
AACT Member-Only Content
You have to be an AACT member to access this content, but good news: anyone can join!
- AACT member benefits »
- Forgot User Name or Password?
Save Your Favorite AACT Resources! ×
Log in or join now to start building your personalized "My Favorites" page. Easily save all the resources you love by logging in and clicking on the star icon next to any resource title.
Plop, Fizz: How to Affect the Rate of a Chemical Reaction Mark as Favorite (51 Favorites)
LAB in Reaction Rate , Reaction Rate , Acid Base Reactions , Kitchen Chemistry . Last updated July 23, 2024.
In this lab, students will react Alka-Seltzer tablets with water. By varying the temperature of the water, particle size of the Alka-Seltzer, and concentration of the Alka-Seltzer they can see the effect on the rate and strength of the chemical reaction.
Grade Level
Middle or high school
NGSS Alignment
This lab will help prepare your students to meet the performance expectations in the following standards:
- MS-PS1-2: Analyze and interpret data on the properties of substances before and after the substances interact to determine if a chemical reaction has occurred.
- MS-PS1-4: Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.
- Using Mathematics and Computational Thinking
- Analyzing and Interpreting Data
- Engaging in Argument from Evidence
By the end of this lab, students should be able to
- understand how changes in temperature, surface area, and concentration can affect the reaction rate.
- make predictions based on the data collected during the experiment.
Chemistry Topics
- Chemical Reactions
- Reaction Rates
Teacher Preparation : 30 minutes
Lesson : One 60 minutes class period (a second period could be used if doing the extension activities)
For the class:
- A large container of room temperature water
- A large container of hot water
- A large container of cold (ice) water
For each lab group:
- One 400 mL beaker
- One mortar and pestle
- 8 Alka-Seltzer tablets
- One thermometer
- One stopwatch or timer
- One cell phone/iPad/computer camera (optional)
- Always wear safety goggles when using chemicals in the lab.
- The final solutions may be discarded into the sink.
- When students complete the lab, instruct them how to clean up their materials.
- Students should wash their hands thoroughly before leaving the lab.
Teacher Notes
- This lab is designed for the students to work in pairs or groups of three.
- The teacher may need to demonstrate how to properly use a mortar and pestle.
- The teacher should demonstrate or show pictures/video of reactions in order to prepare students for how to determine the rating of the strength of a chemical reaction. This could be done with a quick demonstration using vinegar and baking soda.
- During the lab, the students could take a picture or video of the reactions to help them compare and rate the strengths of the various reactions.
- Generic brand antacid effervescent tablets can be substituted for Alka-Seltzer tablets.
- This activity connects well with the Reaction Rate activity in the AACT resource library.
- At the end of the lab, students will be asked how to determine the time of reaction for a value they did not measure. This will involve using the graph to determine where the value on the x-axis meets the best fit line. Students will need to know how to draw a line of best fit. Consider having students complete the graphing simulation before this activity if needed.
- Using the three reaction rate variables, design a way to create the fastest and most vigorous reaction. Describe your method.
- Using the three reaction rate variables, design a way to create the slowest and least vigorous reaction. Describe your method.
- Using the three reaction rate variables, design a way to create a relatively slow and moderately vigorous reaction. Describe your method.
Try each of your ideas and share your results with the class.
For the Student
A chemical reaction is a process where one or more substances (reactants) are chemically changed into one or more new substances (products). In industry, companies try to control the rate of chemical reactions to make them useful and safe. There are several ways to affect how quickly the reaction occurs. We will investigate three of these factors: temperature, particle size (surface area), and the amount of the reactants (concentration).
How do temperature, surface area, and concentration affect the rate of a chemical reaction?
Prelab Questions
- What affect do you think increasing the temperature of one of the reactants will have on the rate of the chemical reaction? Why do you think this?
- What affect do you think increasing the surface area (decreasing the particle size) of one of the reactants will have on the rate of the chemical reaction? Why do you think this?
- What affect do you think increasing the concentration (how much) of one of the reactants will have on the rate of the chemical reaction? Why do you think this?
- Room temperature water
- Cold (ice) water
- Cell phone camera (optional)
- Follow teacher instructions for how to clean up your materials.
- Wash your hands thoroughly before leaving the lab.
The Effect of Temperature:
- Pour 300mL of room temperature water into the 400mL beaker. This will be your control.
- Place the thermometer into the center of the water.
- Once the temperature reading stabilizes, record the temperature in the data table below.
- Get ready to start the stopwatch/timer.
- Start the timer as you drop one Alka-Seltzer tablet into the water.
- Time how long it takes the tablet to finish visibly reacting with the water.
- Record the time (in seconds) in the data table below.
- Also rate the strength of the reaction on a scale of 0 – 5, with 0 being no reaction and 5 being a reaction that would overflow the beaker. You may use a camera to photograph or record a video of the reaction to help in your decision.
- Record the strength of the reaction in the data table below.
- Rinse out the beaker thoroughly with water.
- Repeat steps 1 – 10 with the cold water.
- Repeat steps 1 – 10 with the hot water.
The Effect of Surface Area:
- Pour 300mL of room temperature water into the 400 L beaker.
- Take one Alka-Seltzer tablet and place it in the mortar.
- Use the pestle to crush the tablet into a fine powder.
- Start the timer as you pour the crushed Alka-Seltzer tablet from the mortar into the water.
- Also rate the strength of the reaction on a scale of 0 – 5, with 0 being no reaction and 5 being a reaction that would overflow the beaker. You may use a camera to photo or record a video of the reaction to help in your decision.
- Repeat steps 1 – 10 but use the mortar and pestle to crush the tablet into larger sized pieces.
- Repeat steps 1 – 10 with one uncrushed tablet (this is the control).
The Effect of Concentration:
- Pour 300mL of room temperature water into the 400mL beaker.
- Break one Alka-Seltzer tablet in half.
- Break one of the half tablets in half again to make it a quarter of a tablet.
- Start the timer as you drop the quarter tablet into the water.
- Repeat steps 1 – 10 with the half tablet.
- Repeat steps 1 – 10 with the whole tablet (this is the control).
| Temperature ( C) | Time (seconds) | Strength Rating (0 – 5) |
| Surface Area | Time (seconds) | Strength Rating (0 – 5) |
| Fine Powder | ||
| Larger Pieces | ||
| Whole Tablet |
| Concentration | Time (seconds) | Strength Rating (0 – 5) |
| Quarter Tablet | ||
| Half Tablet | ||
| Whole Tablet |
- On graph paper, plot temperature vs. time to make a line graph . The x-axis should be temperature while the y-axis should be time. Create a line of best fit for the points.
- On graph paper, make a bar graph of the temperature vs. strength. The x-axis should be temperature while the y-axis should be strength.
- On graph paper, make a bar graph of the surface area vs. time. The x-axis should be surface area while the y-axis should be time.
- On graph paper, make a bar graph of the surface area vs. strength. The x-axis should be surface area while the y-axis should be strength.
- On graph paper, plot concentration vs. time to make a line graph. The x-axis should be concentration while the y-axis should be time. Create a line of best fit for the points.
- On graph paper, make a bar graph of the concentration vs. strength. The x-axis should be temperature while the y-axis should be strength.
Write an analysis for each of the variables that we investigated. Write your analysis in paragraph form using the CER format by making a claim (C) and supporting the claim with evidence (E) from your observations, data tables, and graphs, as well as reasoning what you know about chemical reactions (R).
Temperature:
Surface Area:
Concentration:
- Using the temperature data and graph, explain how you can predict the reaction rate of a temperature that is between what you tested and the room temperature water. Use actual temperature values and times in your explanation.
- Using the concentration data and graph, explain how you can predict the reaction rate of a concentration that is different than what was tested. Use actual concentration amounts and times in your explanation.

Rates of Reaction Laboratory Experiment
Rates of reactions.
- Explain how concentration, temperature, catalysts, and surface area affect the rate of a reaction.
- Predict relative reaction rates based on variations in concentration, temperature, and surface area.
- Draw conclusions based on experimental data.
- Determine if a chemical reaction has occurred.
- Perform and record laboratory measurements using common laboratory equipment.
- Explain the importance of collecting accurate experimental data.
Related Textbook
Please read chapter 8 of your textbook before beginning this lab. The textbook provides terms, concepts, and other important background information to help you complete this assignment.
Introduction
Chemical reactions can occur at different speeds, or rates of reactions. Several factors influence the rate of chemical reactions. These factors include:
- Concentration of the Reactants
- Temperature
- Surface Area
In the body, special proteins called enzymes catalyze reactions that would not normally occur at biological temperatures. Catalysts lower the amount of energy, or activation energy , needed to start a chemical reaction. Hormones secreted by various parts of the endocrine system help to regulate various bodily functions, including the rate of reactions in the cell.
A note on surface area: as something is broken into smaller pieces, the overall surface area of the item increases. This is because more surface is exposed for chemicals to react with it. This means that a whole tablet of Alka Seltzer ® has less surface area than a tablet that is broken into smaller pieces. A crushed tablet will have more surface area than a tablet that has been broken into pieces.
In this experiment, you will perform several different reactions and make predictions on how the concentration, temperature, and surface area influence the reaction rate. Be sure to read the information covering reaction rates in your textbook after completing this laboratory experiment; you will be tested over that information on the lab final exam.
You will organize your data for this lab into a data table. Click below for a copy of the data table you will need for this lab. It will download to your computer. If you have trouble downloading it, check to ensure blockers are disabled.
The Rates of Reaction Data Table
Experimental Procedure
Chemicals and Supplies
Alka Seltzer ® (6 tablets, make sure this is regular Alka Seltzer ® and not cold and flu)
Sodium bicarbonate (baking soda), NaHCO 3
Acetic Acid (vinegar), HC 2 H 3 O 2
Digital Balance
9 glasses (drinking glasses are fine)
Paper cupcake liners (weigh boats)
Digital Thermometer
100mL graduated cylinder
Timer with a Second Hand
Pan, Kettle, or microwave-safe container (for heating water)
DI water (distilled or deionized water available at your local grocery story)
PART A: Effect of Temperature on the Rate of Reaction
- Obtain 3 clean and dry glasses. Add 100mL of ice water (less than 5ºC) to one glass, 100mL of room temperature water to the second glass, and 100mL of hot water (more than 50ºC) to the third glass. Record the temperature of each glass of water next to the type of water in the glass on the data table.
- Obtain 3 whole Alka Seltzer ® Drop one tablet in each glass simultaneously.
- Observe the reactions and record what you see happen on the data table provided. Note which tablet completely dissolved first, second, and third. Record this information on your data table under the column titled “Relative Reaction Rate.”
PART B: Effect of Surface Area on the Rate of Reaction
- Obtain 3 clean and dry glasses. Add 100mL of DI water to each glass. Record the temperature of each amount of DI water on the data table. The water in each glass should be within 1ºC of each other. If the temperature of the 3 glasses varies more than 1ºC, allow them to sit for a while until they are all within 1ºC of each other.
- Obtain 3 Alka Seltzer ® Carefully cut one of the tablets into quarters (4 equal-size pieces). Using the 2 spoons, carefully crush one of the tablets into a powder, collecting the pieces in a paper cupcake liner. Leave the third tablet in one piece.
- Add the whole tablet to one glass and record the time it takes for the tablet to fully react with the water. Record the time on the data sheet provided.
- Repeat step 3 using the broken and crushed tablets, placing each in a different glass.
- Record your observations on the data table. Note which tablet completely dissolved first, second, and third. The tablet with the shortest reaction time has the fastest reaction rate. Record this information on your data table under the column titled “Relative Reaction Rate.”
PART C: Effect of Concentration on Reaction Rate
- To Glass #1, add 100mL of acetic acid. This glass will have the highest concentration (amount) of acetic acid.
- To Glass #2, add 70mL of acetic acid and 30mL of DI water. Carefully swirl the glass to mix the water and acetic acid. This glass will have the second-highest concentration of acetic acid.
- To Glass #3, add no acetic acid and 100mL of DI water. This glass will have the lowest concentration of acetic acid.
- Record the temperature of the solutions in the three glasses. The solutions in each glass should be within 1ºC of each other. If the temperature of the 3 glasses varies more than 1ºC, allow them to sit for a while until they are all within 1ºC of each other.
- Using the digital balance, obtain a 1g portion of sodium bicarbonate.
NOTE: To properly measure this amount of sodium bicarbonate, place a weigh boat on the digital balance and “TARE” the balance according to the directions provided with your balance. This tells the balance to ignore the mass of the weigh boat and only measure the mass of the chemical. Next, use a spatula to add 1g of sodium bicarbonate to the weigh boat.
Measuring slightly more or less than 1g of the chemical is okay. Just be sure to write down exactly how much of the chemical you use. Be sure to write down all of the numbers that appear on the balance, even if it’s a zero. This is true of all of the experiments we will conduct in this course.
- Repeat step #3 twice more. When you are done, you should have three 1g portions of sodium bicarbonate.
- Add one portion of sodium bicarbonate to Glass #1 and record the time it takes for the reaction to stop. Record the time on the data table.
- Repeat step #5 for Glass #2 and Glass #3.
- Record your observations on the data table. Note which reaction finished first, second, and third. The glass with the shortest reaction time has the fastest reaction rate. Record this information on your data table under the column titled “Relative Reaction Rate.”
Waste Disposal
- All solutions from this lab can be disposed of down the sink. Flush the sink with warm water after disposing of the chemicals.
- Wash any other glassware used with soap and water once the experiment is complete.
Once you have completed this experiment and recorded your data:
- Submit your completed data table for this lab to the assignment folder on Brightspace. You will receive a zero for this assignment if the data table is incomplete and/or not submitted.
- Complete the post-lab questions for this experiment (provided below) and submit them to the assignment folder on Brightspace. Your assignment can be neatly handwritten or typed using a program such as MSWord or Google Docs. If using a word processing program, please save your file as a .docx or .pdf file before submitting it to Brightspace. Please number your answers to each question so I can clearly and easily follow your work. This assignment is worth 10 points.
- There are no photos of the experiment required for this lab. You just need to complete and submit the data table and post-lab questions.
Rates of Reactions Post-Lab Questions
- Based on your observations, how does concentration affect reaction rates? Do your experimental findings agree with the information provided in your textbook? If not, indicate what error(s) might have happened. (2 points)
- Based on your observations, how does surface area affect reaction rates? Do your experimental findings agree with the information provided in your textbook? If not, indicate what error(s) might have happened. (2 points)
- Based on your observations, how does temperature affect reaction rates? Do your experimental findings agree with the information provided in your textbook? If not, indicate what error(s) might have happened. (2 points)
- Magnesium metal is placed in three test tubes containing hydrochloric acid. Test tube #1 contains 1M HCl, test tube #2 contains 0.1M HCl, and test tube #3 contains 6M HCl. The bigger the number in front of the M, the more concentrated the solution. Which test tube will have the slowest rate of reaction? Why? (1.5 points)
- A glow stick is added to a glass of ice-cold water, a glass of room-temperature water, and a glass of near-boiling water. Which stick will glow the brightest? Why? (1.5 points)
- What is the purpose of enzymes in the body? How do they regulate rates of reaction? (i.e., do they change the body’s temperature, affect chemical concentration, etc.). (1 point)
This page was created on July 6, 2023, and last updated on August 3, 2023.
© Catherine Haslag 2023. All Rights Reserved.


Exploring Surface Area
In this lesson we will discuss how to describe the amount of space and object takes up and review the definitions of area, volume, and surface area. Understanding the amount of surface area on an object is important to material scientists since the surface of a material can affect the rate of reactions and other material properties such as conduction of electricity, emission of light
Essential Question:
What is surface area and what material properties are affected by surface area?
Vocabulary:
Colloquial description of vocab.
- Volume – the amount of space an object occupies
- Surface area – the amount of outermost part of a material
- Defects – a change from the normal pattern in a crystal
Background:
Surface area can play an important part in both material properties and reaction kinetics. In this lesson, we want students to learn (1) the difference between surface area and volume (2) how to increase surface area and (3) why surface area is important to how fast a reaction occurs. Additionally, we want students to understand that surfaces can have a large amounts of defects which can prevent the materials from performing well.
Research Connection:
Surface area can play an important role chemical reactions and material properties. For reactions, having a larger surface area increases the amount of space available for the reaction to occur. Additionally, materials with large amounts of defects on the surface of the material can lose their properties such as conduction of light emission.
NGSS Standards:
| Standard Number | Standard text |
| MS-PS1-2 | Analyze and interpret data on the properties of substances before and after the substance interact to determine if a chemical reaction has occurred. |
- Alka-seltzer tablets
- Plastic cups
- Mortar and pestle
First, review with students how last week they demonstrated how the thickness of materials can affect how it interacts with its surroundings – i.e. nail polish can separate light into different colors. Similarly, the amount of surface area can change properties of materials.
We want to teach students the difference between surface area and volume and that the same volume of material can have different surface areas. A good way to demonstrate this is to have the students stand and form a square as shown below with the students represented with “X”.
X X X X X
The students have now created an object with a certain volume. To draw a connection between volume and amount of material, determine the total number of students and say that this represents the “volume” of our objects.
Next, we want to figure out the surface area of our object. Have the students raise their hand if they are on the outside of the square. These students represent the surface are of the object.
To show that we change the surface area of an object without changing the volume of an object, we want to rearrange the students into a rectangle.
X X X X X X X X X X
Another way to change the surface area is to split an object into smaller pieces. The overall amount of the object remains the same, but the amount of the object on the surface increases. You can show this by having the students break into smaller rectangles.
We have now demonstrated the difference between surface area and volume and how you can change the surface area without changing the volume of a material.
Now, we want to explore the affects of surface area on the rate of reactions. We will do this by having the students observe the differences in how quickly an alka-seltzer tablet dissolves when it is crushed vs when it is whole. Before beginning the experiment, we want students to consider the scientific method – (1) Ask a question (2) construct a hypothesis (3) test with an experiment (4) analyze data (5) determine a conclusion.
The question for this experiment is – Does the surface area affect how quickly the alka-seltzer tablet dissolves?
To test this, students will have two cups in front of them. One cup will have a crushed tablet (done beforehand by volunteers using mortar and pestle) and the other tablet will have a whole tablet.
Ask the students the following questions:
- What factors could affect how quickly the tablets dissolve? Let the students brainstorm, but help them along if needed – they should be able to come up with temperature, amount of water, and surface area.
- Which tablet has the most surface area? Crushed tablet
- How do we control the other factors mentioned? Same water source, keep the level of water the same between the two cups.
- What do they think will happen when they add water? Bubbles will form as the tablet dissolves
- How can you tell which is reacting faster? Time how long it takes to dissolve, how many bubbles are released.
- Which tablet do they think will dissolve faster and why?
Once the students have designed their experiment and come up with their hypothesis, allow them to add water to cups and observe their reactions. Engage with the students as they observe and have them explain how the experiment is proving or disproving their hypothesis.
Once the tablets are dissolve, have the students discuss their results and talk about how surface area can affect the rate of reactions by allowing more sites for the reaction to occur. This can be tied into electrode materials, catalysts, or other research interests.
For the second part of the lesson, we want students to understand that the surface of a material is a place that allows for a lot of material defects . A defect is any change in the pattern of a material, and we can demonstrate defects by having the students form into the square again.
Once the students are in a square, have on of the student from the center try to move out of the square without touching anyone else (the students might have to pack closer together for this). This should be hard for someone inside but easy for someone on the outside of the square. Explain to the students that one type of defect is having a missing piece.
Another type of defect is when a piece is out of place, this can be demonstrated by having a few students step out of their normal place.
To demonstrate how these defects impact the properties of the material, we are going to simulate conduction by passing a balloon through the material. However, if the student not in the normal position then they can not pass the balloon. Have the students repeat this exercise with different amounts of “defects” to show that as the amount of defects increases, the conduction decreases.
- How does surface area impact the rate of reactions?
- How can you increase surface area on a material?
- What happens if the surface of a material has defects? Describe some types of defects in a material?
- What are other applications where surface area is important?
Mentos and Diet Coke Video
Student Worksheet – Surface Area Lesson
Name: ________________________
- What method listed below will increase the surface area on a material?
- Place the material in water
- Break the material into smaller pieces
- Freeze the material
- Heat the material
- Temperature of the water
- Amount of the water
- Number of students watching the reaction
- Adding additional acid to the reaction
- Running a lap around the classroom before starting the reaction
- Write down your hypothesis on how surface area will affect how quickly the acid and base react?
- Record the times below for how long it took for the acid and base to completely react?
Crushed Tablet: Full Tablet:
- Circle the things you observed in the reaction between the crushed alka seltzer and the full alka seltzer tablet?
- The full tablet reacted faster than the crushed tablet
- The crushed tablet reacted faster than the full tablet
- Both tablets reacted at the same speed
- Does the temperature of the water affect the speed of the reactions?
- Does lemon juice affect the speed of the reactions?
- Does more water affect the speed of the reactions?
- Does less water affect the speed of the reactions?
- Record the times below for how long it took for the acid and base to completely react with the different conditions selected above?

Effect of ridge tillage with rice-rape rotation on the root system and yield of crops
- Research Article
- Published: 27 September 2024
Cite this article

- Wenqi Zhang 1 , 2 ,
- Lanting Li 1 ,
- Juan Liu 4 ,
- Fangxin Chen 1 , 2 ,
- Jiupai Ni 1 , 2 ,
- Chaofu Wei 1 , 2 &
- Shouqin Zhong ORCID: orcid.org/0000-0001-9988-9441 1 , 2
Background and aims
Ridge tillage with rice-rape rotation is an important paddy-upland rotation planting pattern in southern China. In order to improve physical environment of soil and increase yield of rice and rape, it is necessary to clarify effects of ridge tillage on root characteristic and yield, which can provide scientific basis for efficient utilization of paddy field.
A field plot experiment was conducted to compare effects of conventional tillage with rice and winter fallow (CK1), conventional tillage with rice-rape rotation (CK2), wide ridge tillage with rice-rape rotation (RT1) and narrow ridge tillage with rice-rape rotation (RT2) on root characteristic and yield of rape and rice.
Ridge tillage (including RT1 and RT2) had positive effects on relevant root indexes and the influence degree was different at different growth stages. For example, at maturity stage, the total root length and surface area of rape treated with RT2 were significantly higher than CK2 by 116.66% and 111.03%. Ridge tillage can significantly improve economic characteristics and yield. Strikingly, compared with CK1 and CK2, the actual yield of rice treated with RT1 was higher by 20.82% and 13.54%.
Conclusions
Ridge tillage can promote root growth, enhance root vitality and increase yield. Narrow ridge tillage (RT2) is more beneficial to the root growth of rape but wide ridge tillage (RT1) is more beneficial to the root growth of rice. The wide ridge is more beneficial to the economic characteristics and yield of rape and rice than narrow ridge.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Explore related subjects
- Environmental Chemistry
Achankeng E, Cornelis W (2023) Conservation tillage effects on European crop yields: a meta-analysis. Field Crops Res 298:108967. https://doi.org/10.1016/j.fcr.2023.108967
Alagbo O, Spaeth M, Saile M, Schumacher M, Gerhards R (2022) Weed Management in Ridge Tillage Systems—A review. Agronomy 12:910. https://doi.org/10.3390/agronomy12040910
Bai Z, Liu H, Wang T, Gong P, Li H, Li L, Xue B, Cao M, Feng J, Xu Y (2021) Effect of smashing Ridge Tillage depth on Soil Water, Salinity, and yield in saline Cotton Fields in South Xinjiang, China. Water 13:3592. https://doi.org/10.3390/w13243592
Article Google Scholar
Chen T, Zhang Y, Fu J, Yang L, Chi Y, Yang K, Wang Y (2021) Effects of tillage methods on soil physical properties and maize growth in a saline–alkali soil. Crop Sci 61:3702–3718. https://doi.org/10.1002/csc2.20589
Article CAS Google Scholar
Eggert M, Schiemann J, Thiele K (2018) Yield performance of Russian dandelion transplants (Taraxacum Koksaghyz L. Rodin) in flat bed and ridge cultivation with different planting densities. Eur J Agron 93:126–134. https://doi.org/10.1016/j.eja.2017.12.003
Fang L (2022) Selection of rice planting mechanization mode in Southwest Lu. Agric Eng Technol 42(18):57–58. https://doi.org/10.16815/j.cnki.11-5436/s.2022.18.022
Gu X, Li Y, Du Y, Ren M, Wu G, Yin M (2016) Effects of fertilization depth on yield, root distribution and nutrient uptake of winter oilseed rape (Brassica napus L). Trans Chin Soc Agric Mach 47(06):120–128
CAS Google Scholar
Guo S, Xia S, Zhu H, Zhang Y, Shi W (2012) Factors influencing collecting amount of rice roots bleeding and investigation on roots vigor after heading. Soils 44(2):308–311. https://doi.org/10.3969/j.issn.0253-9829.2012.02.021
Hakim RO, Kinama JM, Kitonyo OM, Chemining’wa GN, Ahmad I (2022) Effect of Tillage Method and Mulch Application on Growth and Yield of Green Gram in Semiarid Kenya. Adv Agric 2022:1–11. https://doi.org/10.1155/2022/4037022
Huang X, Xu H, Zhang S, Li W, Luo C, Deng Y (2020) Design and experiment of a device for rapeseed strip aerial seeding. Trans Chin Soc Agric Eng 36(5):78–87. https://doi.org/10.11975/j.issn.1002-6819.2020.05.009
Jin Z, Liao W, Mu Y, Li Y, Nie L (2023) Integrated assessment of water footprint and energy production efficiency in different rice-rape rotation systems. Energy 266:126535. https://doi.org/10.1016/j.energy.2022.126535
Kan Y, Liu Y, Li M, Xing G, Ma T, Sun S (2024) Ridge planting can increase the yield of daylily and improve the rhizosphere soil environment. J Shanxi Agric Univ 1–12. https://doi.org/10.13842/j.cnki.issn1671-8151.202403001
Kuai J, Li Z, Wang B, Liu F, Ye J, Zhou G (2021) Effects of density and row spacing on seedling traits of rapeseed and seed yield. Scientia Agricultura Sinica 54(11):2319–2332. https://doi.org/10.3864/j.issn.0578-1752.2021.11.006
Li B, Chen X, Shi X, Liu J, Wei Y, Xiong F (2021) Effects of Ridge Tillage and Straw Mulching on Cultivation the Fresh Faba beans. Agronomy 11:1054. https://doi.org/10.3390/agronomy11061054
Li L, Li J, Wei C, Yang C, Zhong S (2022) Effect of mechanized Ridge Tillage with Rice-rape rotation on Paddy Soil structure. Agriculture 12:2147. https://doi.org/10.3390/agriculture12122147
Liang T, Chen L, Tang M, Wang Q, Zhang X, Lv R (2015) Effects of different water and nitrogen treatments on root growth and yield for rice. J South Agric 46(07):1184–1189. https://doi.org/10.3969/j:issn.2095-1191.2015.7.1184
Lu Y, Wang Q, Yin X, Li Q, Wei L, Zhang X (2020) Rice cultivation techniques of dry direct seeding by mechanical ridge tillage. Hybrid Rice 35(5):49–52. https://doi.org/10.16267/j.cnki.1005-3956.20191217.302
Luo M, Guo L, Fei K, Zhang T, Li C, Ma Y (2022) Cultivated land quality: improving technologies and their application. Chin Agric Sci Bull 38(21):76–81. https://doi.org/10.11924/j.issn.1000-6850.casb2021-0769
Ma X (2023) Cultivation technology of oilseed rape and measures to improve planting efficiency. Seed Sci Technol 41(15):35–37. https://doi.org/10.19904/j.cnki.cn14-1160/s.2023.15.011
Materechera H R M-B SA (1997) Soil penetration resistance, root growth and yield of maize as influenced by tillage system on ridges in Malawi. Soil Tillage Res 41:13–14. https://doi.org/10.1016/S0167-1987(96)01086-0
Ponjican OO, Bajkin AM, Jacimovic GP, Tomic MD, Savin LD, Dedovic NM, Simikic MD (2012) Tillage quality affecting physical characteristics, number of plants and carrot root yield under flat and ridge cultivation. J Food Agric Environ 10:304–311
Qin C, Wright AL, Ma L, He X, Xie D, Jiang X (2020) Improving nitrogen-use efficiency by using ridge tillage in rice paddy soils. Soil Use Manag 38:528–536. https://doi.org/10.1111/sum.12675
Ren B, Dong S, Liu P, Zhao B, Zhang J (2016) Ridge tillage improves plant growth and grain yield of waterlogged summer maize. Agric Water Manage 177:392–399. https://doi.org/10.1016/j.agwat.2016.08.033
Salem HM, Meselhy A, Elhagarey M, Ali AM, Wu W (2022) Soil erosion control and wheat productivity are improved by a developed ridge-furrow and reservoir tillage systems. Arch Agron Soil Sci 68:273–282. https://doi.org/10.1080/03650340.2020.1832655
Saurabh K, Kumar R, Mishra JS, Singh AK, Mondal S, Meena RS, Choudhary JS, Biswas AK, Kumar M, Roy HS, Singh NR, Yadav SK, Upadhyaya A, Hans H, Jeet P, Sundaram PK, Raman RK (2022) Sustainable intensification of rice fallows with oilseeds and pulses: effects on soil aggregation, organic carbon dynamics, and crop productivity in eastern Indo-Gangetic plains. Sustainability 14:11056. https://doi.org/10.3390/su141711056
Terence PM, Murray HM, Doug Y (1999) Mycorrhizae, crop growth, and crop phosphorus nutrition in maize-soybean rotations given various tillage treatments. Plant Soil 210:33–42. https://doi.org/10.1023/A:1004633512450
Uga Y, Sugimoto K, Ogawa S, Rane J, Ishitani M, Hara N, Kitomi Y, Inukai Y, Ono K, Kanno N, Inoue H, Takehisa H, Motoyama R, Nagamura Y, Wu J, Matsumoto T, Takai T, Okuno K, Yano M (2013) Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat Genet 45:1097–1102. https://doi.org/10.1038/ng.2725
Article CAS PubMed Google Scholar
van Ittersum MK, Cassman KG, Grassini P, Wolf J, Tittonell P, Hochman Z (2013) Yield gap analysis with local to global relevance—A review. Field Crops Res 143:4–17. https://doi.org/10.1016/j.fcr.2012.09.009
Wang L, Guo H, Guo H, Liu F, Hui X, Liu Z, Jia X (2023) Effects of ridge planting on tuber yield of helianthus tuberosus in saline-alkali land of the Yellow River Delta. Chin Agric Sci Bull 39(13):13–18. https://doi.org/10.11924/j.issn.1000-6850.casb2022-0350
Wang J, Pan Z, Pan F, He D, Pan Y, Han G, Huang N, Zhang Z, Yin W, Zhang J, Peng R, Wang Z (2020) The regional water-conserving and yield-increasing characteristics and suitability of soil tillage practices in Northern China. Agric Water Manage 228:105883. https://doi.org/10.1016/j.agwat.2019.105883
Wei L, Yin X, Wang Q, Lu Y, Li Q, Zhang X (2021) A preliminary study. on the Production Technology of Direct-seeding Medium Rice—Ratoon Rice with Mechanical Ridge Planting. HYBRID RICE 36(04):50–53. https://doi.org/10.16267/j.cnki.1005-3956.20201014.312
Wu J, Zhang S, Gu D, Gu K (2021) Effects of different straw return methods on rice yield and quality of rice and wheat on an annual basis. China Rice 27(05):79–83. https://doi.org/10.3969/j.issn.1006-8082.2021.05.017
Xia Y, Li H, Wang Z, Fan M, Zhao H, Meng F, Ren Y (2023) Effects of tillage methods on rice quality and yield and soil organic matters in regions of cold climate in China. Fujian J Agricultural Sci 38(05):530–536. https://doi.org/10.19303/j.issn.1008-0384.2023.05.003
Xie J, Yin X, Wei L, Wang Z, Li Q (2023) Effects of control irrigation on grain yield and greenhouse gas emissions in ridge cultivation direct-seeding paddy field. Scientia Agricultura Sinica 56(4):697–710. https://doi.org/10.3864/j.issn.0578-1752.2023.04.009
Xiong J, Chen H, Xiang J, Zhang Y, Wang Y, Wang Z, Hu G, Zhang Y (2021) Effects of different nitrogen application levels on the growth and yield of mechanically inserted and mulched mechanically inserted rice. China Rice 27(05):50–53. https://doi.org/10.3969/j.issn.1006-8082.2021.05.011
Xu G, Ji H, Pan J, Fan L (2012) Exploration of rice seed test methods. China Agricultural Technol Ext 28(06):16–17. https://doi.org/10.3969/j.issn.1002-381X.2012.06.008
Yang D (1990) Effects of semi-arid cultivation-ridge tillage and different widths on rice yield. Tillage Cultivation 02(2):36–80
Google Scholar
Yao Y (2015) Effects of ridge tillage on photosynthesis and root characters of rice. Chil J Agricultural Res 75:35–41. https://doi.org/10.4067/s0718-58392015000100005
Ye S (2004) Discussion on the experimental teaching material system of plant physiology and biochemistry in agricultural colleges. Plant Physiol J 04:487–488. https://doi.org/10.13592/j.cnki.ppj.2004.04.038
Yousaf M, Li X, Zhang Z, Ren T, Cong R, Ata-Ul-Karim ST, Fahad S, Shah AN, Lu J (2016) Nitrogen fertilizer management for enhancing crop productivity and nitrogen use efficiency in a rice-oilseed rape rotation system in China. Front Plant Sci 7. https://doi.org/10.3389/fpls.2016.01496
Yu J, Du J, Zhou J, Hu J, Tao G (2020) Research and application of high-yield and high-efficiency cultivation technology of rice-oil rotation. China South Agricultural Mach 51(23):26
Zaffar M, Lu S-G (2015) Pore size distribution of Clayey soils and its correlation with Soil Organic Matter. Pedosphere 25:240–249. https://doi.org/10.1016/s1002-0160(15)60009-1
Zeng Y, Fang Z (2021) Status and development strategy of full mechanization of peanut in the hilly area of south China. Agricultural Eng Equip 48(06):04–09
Zhang J, Frielinghaus M, Tian G, Lobb D (2004) Ridge and contour tillage effects on soil erosion from steep hillslopes in the Sichuan Basin,China. Journai soil Water Conserv 59:277–284
Zheng W, Ye C, Xiao G, Chen M, Li Y, Huang T, Xiao X, Liu X, Zhu C (2015) Effects of symbiotic period on seedling traits and yield components of interplanting rapeseed in rice. Scientia Agricultura Sinica 48(21):4254–4263. https://doi.org/10.3864/j.issn.0578-1752.2015.21.006
Zhou S, Li C, Chang S, Lian Y, Liu K (2000) Effects of ridge culture on summer maize ecological environment and growth development. J Henan Agricultural Univ 34(03):206–209. https://doi.org/10.16445/j.cnki.1000-2340.2000.03.002
Download references
Acknowledgements
This work was financially supported by the National Nature Science Foundation of China (Grant Number: 42307423), the National Key R&D Program of China (Grant Number: 2023YFD1901201), the General Project of Chongqing Natural Science Foundation (Grant Number: CSTB2022NSCQ-MSX0446), the Science and Technology Research Plan of Chongqing Municipal Education Commission (Grant Number: KJQN202100542) and the special fund for youth team of the Southwest University (Grant Number: Swu-xjpy202303). We gratefully acknowledge Dr. Chengsheng Ni (College of Resources and Environments, Southwest University) made plentiful valuable comments for the manuscript.
Author information
Authors and affiliations.
College of Resources and Environment, Southwest University, No.2 Tiansheng Road, BeiBei District, Chongqing, 400715, PR China
Wenqi Zhang, Lanting Li, Fangxin Chen, Jiupai Ni, Chaofu Wei & Shouqin Zhong
Key Laboratory of Arable Land Conservation (Southwest China), Ministry of Agriculture and Rural Affairs, Chongqing, 400715, China
Wenqi Zhang, Fangxin Chen, Jiupai Ni, Chaofu Wei & Shouqin Zhong
Chongqing Municipal Planning and Natural Resources Investigation and Monitoring Institute, Chongqing, 401122, China
College of Geography and Tourism, Chongqing Normal University, Chongqing, 401331, China
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Shouqin Zhong .
Ethics declarations
Competing interests.
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Jairo A. Palta.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
(XLSX 19.6 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Zhang, W., Li, L., Liu, B. et al. Effect of ridge tillage with rice-rape rotation on the root system and yield of crops. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06914-1
Download citation
Received : 29 March 2024
Accepted : 15 August 2024
Published : 27 September 2024
DOI : https://doi.org/10.1007/s11104-024-06914-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Ridge tillage
- Rice-rape rotation
- Root morphology
- Root activity
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
Take the fourth tablet and ground it into a powder. To do this, put the tablet to be ground inside a clean, folded piece of paper. Place the folded paper on a solid surface and use a spoon to ...
Given the Alka-Seltzer mass and time values, let students calculate and record each average reaction rate in the data table provided in their worksheet. Let the students make a bar graph showing the average reaction rate, in grams/second, (y-axis) vs. surface area on the x-axis. As they cannot calculate the actual surface area of the tablet ...
Set all four pieces aside. Take the fourth tablet and ground it into a powder. To do this, put the tablet to be ground inside a clean, folded piece of paper. Place the folded paper on a solid surface and use a hammer or metal spoon to carefully crush the tablet into a powder. Keep the powder folded into the paper and set it aside.
Add a tablet and record the time it takes for the tablet to react. Repeat 1-2 with room temperature water, then with hot water heated to 70 degrees C using a hot plate. The Effect of Surface Area on Rate of Reaction. Prepare three different sizes of the Alka Seltzer tablets: A whole tablet; A tablet broken into quarters
This explained my results. For my whole tablet test, the average was 2:40. For the chunks test, the average was 1:44. Lastly for the crushed test, the average was 1:18. Overall, the third experiment was a success. It has proven that surface area is in fact a factor in the rate of reaction. The results show a change in over 30% in the experiment ...
The main ingredients of Alka-Seltzer tablets are aspirin, citric acid, and sodium bicarbonate (NaHCO 3). When sodium bicarbonate dissolves in water, it dissociates (splits apart) into sodium (Na +) and bicarbonate (HCO 3−) ions. The bicarbonate reacts with hydrogen ions (H +) from the citric acid to form carbon dioxide and water.
This video is an introduction for students to our "Reaction Rates: When Surface Area Matters!" lesson plan, where they investigate the effect of surface area...
The water reacts with the tablet to create bubbles full of carbon dioxide gas. Chemistry has a lot to do with chemical reactions, but it also studies properties of materials. The property at work in this experiment is surface area. Small things have more surface area for their volume than larger things do.
The Effect of Surface Area: Pour 300mL of room temperature water into the 400 L beaker. Take one Alka-Seltzer tablet and place it in the mortar. Use the pestle to crush the tablet into a fine powder. Get ready to start the stopwatch/timer. Start the timer as you pour the crushed Alka-Seltzer tablet from the mortar into the water.
A note on surface area: as something is broken into smaller pieces, the overall surface area of the item increases. This is because more surface is exposed for chemicals to react with it. This means that a whole tablet of Alka Seltzer ® has less surface area than a tablet that is broken into smaller pieces. A crushed tablet will have more ...
Hazel and Emilia demonstrate how to investigate the effect of surface area on the rate of reaction. Different sized marble chips (calcium carbonate) are reac...
Calculate its approximate surface area. Make the same calculation for surface area of the quartered tablet. Does the increase in reaction rate you observed with the quartered tablet approximately correspond to the increase in surface area? If not, propose an explanation. Q3: Most fizzy tablet remedies direct the user to dissolve two tablets in ...
The question for this experiment is - Does the surface area affect how quickly the alka-seltzer tablet dissolves? To test this, students will have two cups in front of them. One cup will have a crushed tablet (done beforehand by volunteers using mortar and pestle) and the other tablet will have a whole tablet.
In order for the reaction shown above to take place, the ingredients in the tablet first have to dissolve. The tablet has a large surface area, so this step should be pretty fast, right? ... Remember that you need to have three whole tablets for this experiment. For each of the four particle sizes, you should repeat the experiment three times ...
Pick up a whole tablet and drop it into the second beaker of water. Time how long it takes to dissolve completely. Discussion: This activity demonstrates how increasing the surface area of an antacid tablet by crushing it into a powder increases the rate in which it dissolves in water. This is a similar situation to the way the thrust of a ...
Part B. Tablet Broken into ~8 Pieces. Place 1 Alka-Seltzer tablet onto a sheet of paper and break into approximately 8 pieces of about equal size. Fill a clear glass with exactly 8 oz./240 mL of room temperature or lukewarm water. Slide broken tablet into the water from the sheet. Measure and record the time to dissolve.
3. determine at least two conditions that affect the reaction rate of Alka-Seltzer® tablets in water. 4. devise a method to measure reaction rate. 5. distinguish between independent and dependent variables in an experiment. 6. design a controlled experiment. 7. construct a graph of temperature versus time (or rate). 8.
With a mean, or average, difference of 38 seconds between the crushed and whole tablets the effect of surface area on the rate of reaction is quite obvious in the outcome of this experiment. This outcome could be easily predicted through the research of theoretical and expected results, as it is obvious that surface area does in fact have.
Increasing the surface area of the solid increases the chances of collision taking place. Imagine a reaction between magnesium metal and a dilute acid like hydrochloric acid. The reaction involves collision between magnesium atoms and hydrogen ions. Increasing the number of collisions per second increases the rate of reaction. You will find ...
Write down your hypothesis about how the surface area of the Alka-Seltzer tablet will affect the reaction rate. Write 1-3 sentences explaining why this is your hypothesis. Follow the steps below to conduct your experiment. 2. Experiment. Experiment 1: Whole Alka-Seltzer tablet. a. Fill the beaker or cup with water up to the top of the masking ...
Chemguide: Core Chemistry 14 - 16. The effect of surface area on the rates of chemical reactions. This page uses the reaction between marble chips and dilute hydrochloric acid to investigate the relationship between the rate of reaction and the surface area of the marble chips. It uses a video which lets you see a commonly done experiment as it ...
Extra: Compare whole Alka-Seltzer tablets to pieces of Alka-Seltzer tablets. If there is a greater surface area (i.e., a tablet is broken up into more pieces), does the same amount of tablet result in the reaction happening faster or slower? Extra: You could turn this activity into a homemade lava lamp! To do this, you will want to use a tall ...
Study area. The field experiment of this study was conducted in the test field of the national monitoring station of soil fertility and fertilizer efficiency on purple soils (30°26′N;106°26′E), with an altitude of 230 m (Fig. 1).The soil type is the neutral purple paddy soil [Anthrosols in the WRB Taxonomy (Zaffar and Lu 2015)] developed on the parent material of the gray-brown-purple ...