- The 25 Most Influential Psychological Experiments in History

While each year thousands and thousands of studies are completed in the many specialty areas of psychology, there are a handful that, over the years, have had a lasting impact in the psychological community as a whole. Some of these were dutifully conducted, keeping within the confines of ethical and practical guidelines. Others pushed the boundaries of human behavior during their psychological experiments and created controversies that still linger to this day. And still others were not designed to be true psychological experiments, but ended up as beacons to the psychological community in proving or disproving theories.
This is a list of the 25 most influential psychological experiments still being taught to psychology students of today.

1. A Class Divided
Study conducted by: jane elliott.
Study Conducted in 1968 in an Iowa classroom

Experiment Details: Jane Elliott’s famous experiment was inspired by the assassination of Dr. Martin Luther King Jr. and the inspirational life that he led. The third grade teacher developed an exercise, or better yet, a psychological experiment, to help her Caucasian students understand the effects of racism and prejudice.
Elliott divided her class into two separate groups: blue-eyed students and brown-eyed students. On the first day, she labeled the blue-eyed group as the superior group and from that point forward they had extra privileges, leaving the brown-eyed children to represent the minority group. She discouraged the groups from interacting and singled out individual students to stress the negative characteristics of the children in the minority group. What this exercise showed was that the children’s behavior changed almost instantaneously. The group of blue-eyed students performed better academically and even began bullying their brown-eyed classmates. The brown-eyed group experienced lower self-confidence and worse academic performance. The next day, she reversed the roles of the two groups and the blue-eyed students became the minority group.
At the end of the experiment, the children were so relieved that they were reported to have embraced one another and agreed that people should not be judged based on outward appearances. This exercise has since been repeated many times with similar outcomes.
For more information click here
2. Asch Conformity Study
Study conducted by: dr. solomon asch.
Study Conducted in 1951 at Swarthmore College

Experiment Details: Dr. Solomon Asch conducted a groundbreaking study that was designed to evaluate a person’s likelihood to conform to a standard when there is pressure to do so.
A group of participants were shown pictures with lines of various lengths and were then asked a simple question: Which line is longest? The tricky part of this study was that in each group only one person was a true participant. The others were actors with a script. Most of the actors were instructed to give the wrong answer. Strangely, the one true participant almost always agreed with the majority, even though they knew they were giving the wrong answer.
The results of this study are important when we study social interactions among individuals in groups. This study is a famous example of the temptation many of us experience to conform to a standard during group situations and it showed that people often care more about being the same as others than they do about being right. It is still recognized as one of the most influential psychological experiments for understanding human behavior.
3. Bobo Doll Experiment
Study conducted by: dr. alburt bandura.
Study Conducted between 1961-1963 at Stanford University

In his groundbreaking study he separated participants into three groups:
- one was exposed to a video of an adult showing aggressive behavior towards a Bobo doll
- another was exposed to video of a passive adult playing with the Bobo doll
- the third formed a control group
Children watched their assigned video and then were sent to a room with the same doll they had seen in the video (with the exception of those in the control group). What the researcher found was that children exposed to the aggressive model were more likely to exhibit aggressive behavior towards the doll themselves. The other groups showed little imitative aggressive behavior. For those children exposed to the aggressive model, the number of derivative physical aggressions shown by the boys was 38.2 and 12.7 for the girls.
The study also showed that boys exhibited more aggression when exposed to aggressive male models than boys exposed to aggressive female models. When exposed to aggressive male models, the number of aggressive instances exhibited by boys averaged 104. This is compared to 48.4 aggressive instances exhibited by boys who were exposed to aggressive female models.
While the results for the girls show similar findings, the results were less drastic. When exposed to aggressive female models, the number of aggressive instances exhibited by girls averaged 57.7. This is compared to 36.3 aggressive instances exhibited by girls who were exposed to aggressive male models. The results concerning gender differences strongly supported Bandura’s secondary prediction that children will be more strongly influenced by same-sex models. The Bobo Doll Experiment showed a groundbreaking way to study human behavior and it’s influences.
4. Car Crash Experiment
Study conducted by: elizabeth loftus and john palmer.
Study Conducted in 1974 at The University of California in Irvine

The participants watched slides of a car accident and were asked to describe what had happened as if they were eyewitnesses to the scene. The participants were put into two groups and each group was questioned using different wording such as “how fast was the car driving at the time of impact?” versus “how fast was the car going when it smashed into the other car?” The experimenters found that the use of different verbs affected the participants’ memories of the accident, showing that memory can be easily distorted.
This research suggests that memory can be easily manipulated by questioning technique. This means that information gathered after the event can merge with original memory causing incorrect recall or reconstructive memory. The addition of false details to a memory of an event is now referred to as confabulation. This concept has very important implications for the questions used in police interviews of eyewitnesses.
5. Cognitive Dissonance Experiment
Study conducted by: leon festinger and james carlsmith.
Study Conducted in 1957 at Stanford University
Experiment Details: The concept of cognitive dissonance refers to a situation involving conflicting:
This conflict produces an inherent feeling of discomfort leading to a change in one of the attitudes, beliefs or behaviors to minimize or eliminate the discomfort and restore balance.
Cognitive dissonance was first investigated by Leon Festinger, after an observational study of a cult that believed that the earth was going to be destroyed by a flood. Out of this study was born an intriguing experiment conducted by Festinger and Carlsmith where participants were asked to perform a series of dull tasks (such as turning pegs in a peg board for an hour). Participant’s initial attitudes toward this task were highly negative.
They were then paid either $1 or $20 to tell a participant waiting in the lobby that the tasks were really interesting. Almost all of the participants agreed to walk into the waiting room and persuade the next participant that the boring experiment would be fun. When the participants were later asked to evaluate the experiment, the participants who were paid only $1 rated the tedious task as more fun and enjoyable than the participants who were paid $20 to lie.
Being paid only $1 is not sufficient incentive for lying and so those who were paid $1 experienced dissonance. They could only overcome that cognitive dissonance by coming to believe that the tasks really were interesting and enjoyable. Being paid $20 provides a reason for turning pegs and there is therefore no dissonance.
6. Fantz’s Looking Chamber
Study conducted by: robert l. fantz.
Study Conducted in 1961 at the University of Illinois
Experiment Details: The study conducted by Robert L. Fantz is among the simplest, yet most important in the field of infant development and vision. In 1961, when this experiment was conducted, there very few ways to study what was going on in the mind of an infant. Fantz realized that the best way was to simply watch the actions and reactions of infants. He understood the fundamental factor that if there is something of interest near humans, they generally look at it.
To test this concept, Fantz set up a display board with two pictures attached. On one was a bulls-eye. On the other was the sketch of a human face. This board was hung in a chamber where a baby could lie safely underneath and see both images. Then, from behind the board, invisible to the baby, he peeked through a hole to watch what the baby looked at. This study showed that a two-month old baby looked twice as much at the human face as it did at the bulls-eye. This suggests that human babies have some powers of pattern and form selection. Before this experiment it was thought that babies looked out onto a chaotic world of which they could make little sense.
7. Hawthorne Effect
Study conducted by: henry a. landsberger.
Study Conducted in 1955 at Hawthorne Works in Chicago, Illinois

Landsberger performed the study by analyzing data from experiments conducted between 1924 and 1932, by Elton Mayo, at the Hawthorne Works near Chicago. The company had commissioned studies to evaluate whether the level of light in a building changed the productivity of the workers. What Mayo found was that the level of light made no difference in productivity. The workers increased their output whenever the amount of light was switched from a low level to a high level, or vice versa.
The researchers noticed a tendency that the workers’ level of efficiency increased when any variable was manipulated. The study showed that the output changed simply because the workers were aware that they were under observation. The conclusion was that the workers felt important because they were pleased to be singled out. They increased productivity as a result. Being singled out was the factor dictating increased productivity, not the changing lighting levels, or any of the other factors that they experimented upon.
The Hawthorne Effect has become one of the hardest inbuilt biases to eliminate or factor into the design of any experiment in psychology and beyond.
8. Kitty Genovese Case
Study conducted by: new york police force.
Study Conducted in 1964 in New York City
Experiment Details: The murder case of Kitty Genovese was never intended to be a psychological experiment, however it ended up having serious implications for the field.
According to a New York Times article, almost 40 neighbors witnessed Kitty Genovese being savagely attacked and murdered in Queens, New York in 1964. Not one neighbor called the police for help. Some reports state that the attacker briefly left the scene and later returned to “finish off” his victim. It was later uncovered that many of these facts were exaggerated. (There were more likely only a dozen witnesses and records show that some calls to police were made).
What this case later become famous for is the “Bystander Effect,” which states that the more bystanders that are present in a social situation, the less likely it is that anyone will step in and help. This effect has led to changes in medicine, psychology and many other areas. One famous example is the way CPR is taught to new learners. All students in CPR courses learn that they must assign one bystander the job of alerting authorities which minimizes the chances of no one calling for assistance.
9. Learned Helplessness Experiment
Study conducted by: martin seligman.
Study Conducted in 1967 at the University of Pennsylvania

Seligman’s experiment involved the ringing of a bell and then the administration of a light shock to a dog. After a number of pairings, the dog reacted to the shock even before it happened. As soon as the dog heard the bell, he reacted as though he’d already been shocked.
During the course of this study something unexpected happened. Each dog was placed in a large crate that was divided down the middle with a low fence. The dog could see and jump over the fence easily. The floor on one side of the fence was electrified, but not on the other side of the fence. Seligman placed each dog on the electrified side and administered a light shock. He expected the dog to jump to the non-shocking side of the fence. In an unexpected turn, the dogs simply laid down.
The hypothesis was that as the dogs learned from the first part of the experiment that there was nothing they could do to avoid the shocks, they gave up in the second part of the experiment. To prove this hypothesis the experimenters brought in a new set of animals and found that dogs with no history in the experiment would jump over the fence.
This condition was described as learned helplessness. A human or animal does not attempt to get out of a negative situation because the past has taught them that they are helpless.
10. Little Albert Experiment
Study conducted by: john b. watson and rosalie rayner.
Study Conducted in 1920 at Johns Hopkins University

The experiment began by placing a white rat in front of the infant, who initially had no fear of the animal. Watson then produced a loud sound by striking a steel bar with a hammer every time little Albert was presented with the rat. After several pairings (the noise and the presentation of the white rat), the boy began to cry and exhibit signs of fear every time the rat appeared in the room. Watson also created similar conditioned reflexes with other common animals and objects (rabbits, Santa beard, etc.) until Albert feared them all.
This study proved that classical conditioning works on humans. One of its most important implications is that adult fears are often connected to early childhood experiences.
11. Magical Number Seven
Study conducted by: george a. miller.
Study Conducted in 1956 at Princeton University
Experiment Details: Frequently referred to as “ Miller’s Law,” the Magical Number Seven experiment purports that the number of objects an average human can hold in working memory is 7 ± 2. This means that the human memory capacity typically includes strings of words or concepts ranging from 5-9. This information on the limits to the capacity for processing information became one of the most highly cited papers in psychology.
The Magical Number Seven Experiment was published in 1956 by cognitive psychologist George A. Miller of Princeton University’s Department of Psychology in Psychological Review . In the article, Miller discussed a concurrence between the limits of one-dimensional absolute judgment and the limits of short-term memory.
In a one-dimensional absolute-judgment task, a person is presented with a number of stimuli that vary on one dimension (such as 10 different tones varying only in pitch). The person responds to each stimulus with a corresponding response (learned before).
Performance is almost perfect up to five or six different stimuli but declines as the number of different stimuli is increased. This means that a human’s maximum performance on one-dimensional absolute judgment can be described as an information store with the maximum capacity of approximately 2 to 3 bits of information There is the ability to distinguish between four and eight alternatives.
12. Pavlov’s Dog Experiment
Study conducted by: ivan pavlov.
Study Conducted in the 1890s at the Military Medical Academy in St. Petersburg, Russia

Pavlov began with the simple idea that there are some things that a dog does not need to learn. He observed that dogs do not learn to salivate when they see food. This reflex is “hard wired” into the dog. This is an unconditioned response (a stimulus-response connection that required no learning).
Pavlov outlined that there are unconditioned responses in the animal by presenting a dog with a bowl of food and then measuring its salivary secretions. In the experiment, Pavlov used a bell as his neutral stimulus. Whenever he gave food to his dogs, he also rang a bell. After a number of repeats of this procedure, he tried the bell on its own. What he found was that the bell on its own now caused an increase in salivation. The dog had learned to associate the bell and the food. This learning created a new behavior. The dog salivated when he heard the bell. Because this response was learned (or conditioned), it is called a conditioned response. The neutral stimulus has become a conditioned stimulus.
This theory came to be known as classical conditioning.
13. Robbers Cave Experiment
Study conducted by: muzafer and carolyn sherif.
Study Conducted in 1954 at the University of Oklahoma
Experiment Details: This experiment, which studied group conflict, is considered by most to be outside the lines of what is considered ethically sound.
In 1954 researchers at the University of Oklahoma assigned 22 eleven- and twelve-year-old boys from similar backgrounds into two groups. The two groups were taken to separate areas of a summer camp facility where they were able to bond as social units. The groups were housed in separate cabins and neither group knew of the other’s existence for an entire week. The boys bonded with their cabin mates during that time. Once the two groups were allowed to have contact, they showed definite signs of prejudice and hostility toward each other even though they had only been given a very short time to develop their social group. To increase the conflict between the groups, the experimenters had them compete against each other in a series of activities. This created even more hostility and eventually the groups refused to eat in the same room. The final phase of the experiment involved turning the rival groups into friends. The fun activities the experimenters had planned like shooting firecrackers and watching movies did not initially work, so they created teamwork exercises where the two groups were forced to collaborate. At the end of the experiment, the boys decided to ride the same bus home, demonstrating that conflict can be resolved and prejudice overcome through cooperation.
Many critics have compared this study to Golding’s Lord of the Flies novel as a classic example of prejudice and conflict resolution.
14. Ross’ False Consensus Effect Study
Study conducted by: lee ross.
Study Conducted in 1977 at Stanford University
Experiment Details: In 1977, a social psychology professor at Stanford University named Lee Ross conducted an experiment that, in lay terms, focuses on how people can incorrectly conclude that others think the same way they do, or form a “false consensus” about the beliefs and preferences of others. Ross conducted the study in order to outline how the “false consensus effect” functions in humans.
Featured Programs
In the first part of the study, participants were asked to read about situations in which a conflict occurred and then were told two alternative ways of responding to the situation. They were asked to do three things:
- Guess which option other people would choose
- Say which option they themselves would choose
- Describe the attributes of the person who would likely choose each of the two options
What the study showed was that most of the subjects believed that other people would do the same as them, regardless of which of the two responses they actually chose themselves. This phenomenon is referred to as the false consensus effect, where an individual thinks that other people think the same way they do when they may not. The second observation coming from this important study is that when participants were asked to describe the attributes of the people who will likely make the choice opposite of their own, they made bold and sometimes negative predictions about the personalities of those who did not share their choice.
15. The Schachter and Singer Experiment on Emotion
Study conducted by: stanley schachter and jerome e. singer.
Study Conducted in 1962 at Columbia University
Experiment Details: In 1962 Schachter and Singer conducted a ground breaking experiment to prove their theory of emotion.
In the study, a group of 184 male participants were injected with epinephrine, a hormone that induces arousal including increased heartbeat, trembling, and rapid breathing. The research participants were told that they were being injected with a new medication to test their eyesight. The first group of participants was informed the possible side effects that the injection might cause while the second group of participants were not. The participants were then placed in a room with someone they thought was another participant, but was actually a confederate in the experiment. The confederate acted in one of two ways: euphoric or angry. Participants who had not been informed about the effects of the injection were more likely to feel either happier or angrier than those who had been informed.
What Schachter and Singer were trying to understand was the ways in which cognition or thoughts influence human emotion. Their study illustrates the importance of how people interpret their physiological states, which form an important component of your emotions. Though their cognitive theory of emotional arousal dominated the field for two decades, it has been criticized for two main reasons: the size of the effect seen in the experiment was not that significant and other researchers had difficulties repeating the experiment.
16. Selective Attention / Invisible Gorilla Experiment
Study conducted by: daniel simons and christopher chabris.
Study Conducted in 1999 at Harvard University
Experiment Details: In 1999 Simons and Chabris conducted their famous awareness test at Harvard University.
Participants in the study were asked to watch a video and count how many passes occurred between basketball players on the white team. The video moves at a moderate pace and keeping track of the passes is a relatively easy task. What most people fail to notice amidst their counting is that in the middle of the test, a man in a gorilla suit walked onto the court and stood in the center before walking off-screen.
The study found that the majority of the subjects did not notice the gorilla at all, proving that humans often overestimate their ability to effectively multi-task. What the study set out to prove is that when people are asked to attend to one task, they focus so strongly on that element that they may miss other important details.
17. Stanford Prison Study
Study conducted by philip zimbardo.
Study Conducted in 1971 at Stanford University

The Stanford Prison Experiment was designed to study behavior of “normal” individuals when assigned a role of prisoner or guard. College students were recruited to participate. They were assigned roles of “guard” or “inmate.” Zimbardo played the role of the warden. The basement of the psychology building was the set of the prison. Great care was taken to make it look and feel as realistic as possible.
The prison guards were told to run a prison for two weeks. They were told not to physically harm any of the inmates during the study. After a few days, the prison guards became very abusive verbally towards the inmates. Many of the prisoners became submissive to those in authority roles. The Stanford Prison Experiment inevitably had to be cancelled because some of the participants displayed troubling signs of breaking down mentally.
Although the experiment was conducted very unethically, many psychologists believe that the findings showed how much human behavior is situational. People will conform to certain roles if the conditions are right. The Stanford Prison Experiment remains one of the most famous psychology experiments of all time.
18. Stanley Milgram Experiment
Study conducted by stanley milgram.
Study Conducted in 1961 at Stanford University
Experiment Details: This 1961 study was conducted by Yale University psychologist Stanley Milgram. It was designed to measure people’s willingness to obey authority figures when instructed to perform acts that conflicted with their morals. The study was based on the premise that humans will inherently take direction from authority figures from very early in life.
Participants were told they were participating in a study on memory. They were asked to watch another person (an actor) do a memory test. They were instructed to press a button that gave an electric shock each time the person got a wrong answer. (The actor did not actually receive the shocks, but pretended they did).
Participants were told to play the role of “teacher” and administer electric shocks to “the learner,” every time they answered a question incorrectly. The experimenters asked the participants to keep increasing the shocks. Most of them obeyed even though the individual completing the memory test appeared to be in great pain. Despite these protests, many participants continued the experiment when the authority figure urged them to. They increased the voltage after each wrong answer until some eventually administered what would be lethal electric shocks.
This experiment showed that humans are conditioned to obey authority and will usually do so even if it goes against their natural morals or common sense.
19. Surrogate Mother Experiment
Study conducted by: harry harlow.
Study Conducted from 1957-1963 at the University of Wisconsin
Experiment Details: In a series of controversial experiments during the late 1950s and early 1960s, Harry Harlow studied the importance of a mother’s love for healthy childhood development.
In order to do this he separated infant rhesus monkeys from their mothers a few hours after birth and left them to be raised by two “surrogate mothers.” One of the surrogates was made of wire with an attached bottle for food. The other was made of soft terrycloth but lacked food. The researcher found that the baby monkeys spent much more time with the cloth mother than the wire mother, thereby proving that affection plays a greater role than sustenance when it comes to childhood development. They also found that the monkeys that spent more time cuddling the soft mother grew up to healthier.
This experiment showed that love, as demonstrated by physical body contact, is a more important aspect of the parent-child bond than the provision of basic needs. These findings also had implications in the attachment between fathers and their infants when the mother is the source of nourishment.
20. The Good Samaritan Experiment
Study conducted by: john darley and daniel batson.
Study Conducted in 1973 at The Princeton Theological Seminary (Researchers were from Princeton University)
Experiment Details: In 1973, an experiment was created by John Darley and Daniel Batson, to investigate the potential causes that underlie altruistic behavior. The researchers set out three hypotheses they wanted to test:
- People thinking about religion and higher principles would be no more inclined to show helping behavior than laymen.
- People in a rush would be much less likely to show helping behavior.
- People who are religious for personal gain would be less likely to help than people who are religious because they want to gain some spiritual and personal insights into the meaning of life.
Student participants were given some religious teaching and instruction. They were then were told to travel from one building to the next. Between the two buildings was a man lying injured and appearing to be in dire need of assistance. The first variable being tested was the degree of urgency impressed upon the subjects, with some being told not to rush and others being informed that speed was of the essence.
The results of the experiment were intriguing, with the haste of the subject proving to be the overriding factor. When the subject was in no hurry, nearly two-thirds of people stopped to lend assistance. When the subject was in a rush, this dropped to one in ten.
People who were on the way to deliver a speech about helping others were nearly twice as likely to help as those delivering other sermons,. This showed that the thoughts of the individual were a factor in determining helping behavior. Religious beliefs did not appear to make much difference on the results. Being religious for personal gain, or as part of a spiritual quest, did not appear to make much of an impact on the amount of helping behavior shown.
21. The Halo Effect Experiment
Study conducted by: richard e. nisbett and timothy decamp wilson.
Study Conducted in 1977 at the University of Michigan
Experiment Details: The Halo Effect states that people generally assume that people who are physically attractive are more likely to:
- be intelligent
- be friendly
- display good judgment
To prove their theory, Nisbett and DeCamp Wilson created a study to prove that people have little awareness of the nature of the Halo Effect. They’re not aware that it influences:
- their personal judgments
- the production of a more complex social behavior
In the experiment, college students were the research participants. They were asked to evaluate a psychology instructor as they view him in a videotaped interview. The students were randomly assigned to one of two groups. Each group was shown one of two different interviews with the same instructor. The instructor is a native French-speaking Belgian who spoke English with a noticeable accent. In the first video, the instructor presented himself as someone:
- respectful of his students’ intelligence and motives
- flexible in his approach to teaching
- enthusiastic about his subject matter
In the second interview, he presented himself as much more unlikable. He was cold and distrustful toward the students and was quite rigid in his teaching style.
After watching the videos, the subjects were asked to rate the lecturer on:
- physical appearance
His mannerisms and accent were kept the same in both versions of videos. The subjects were asked to rate the professor on an 8-point scale ranging from “like extremely” to “dislike extremely.” Subjects were also told that the researchers were interested in knowing “how much their liking for the teacher influenced the ratings they just made.” Other subjects were asked to identify how much the characteristics they just rated influenced their liking of the teacher.
After responding to the questionnaire, the respondents were puzzled about their reactions to the videotapes and to the questionnaire items. The students had no idea why they gave one lecturer higher ratings. Most said that how much they liked the lecturer had not affected their evaluation of his individual characteristics at all.
The interesting thing about this study is that people can understand the phenomenon, but they are unaware when it is occurring. Without realizing it, humans make judgments. Even when it is pointed out, they may still deny that it is a product of the halo effect phenomenon.
22. The Marshmallow Test
Study conducted by: walter mischel.
Study Conducted in 1972 at Stanford University

In his 1972 Marshmallow Experiment, children ages four to six were taken into a room where a marshmallow was placed in front of them on a table. Before leaving each of the children alone in the room, the experimenter informed them that they would receive a second marshmallow if the first one was still on the table after they returned in 15 minutes. The examiner recorded how long each child resisted eating the marshmallow and noted whether it correlated with the child’s success in adulthood. A small number of the 600 children ate the marshmallow immediately and one-third delayed gratification long enough to receive the second marshmallow.
In follow-up studies, Mischel found that those who deferred gratification were significantly more competent and received higher SAT scores than their peers. This characteristic likely remains with a person for life. While this study seems simplistic, the findings outline some of the foundational differences in individual traits that can predict success.
23. The Monster Study
Study conducted by: wendell johnson.
Study Conducted in 1939 at the University of Iowa
Experiment Details: The Monster Study received this negative title due to the unethical methods that were used to determine the effects of positive and negative speech therapy on children.
Wendell Johnson of the University of Iowa selected 22 orphaned children, some with stutters and some without. The children were in two groups. The group of children with stutters was placed in positive speech therapy, where they were praised for their fluency. The non-stutterers were placed in negative speech therapy, where they were disparaged for every mistake in grammar that they made.
As a result of the experiment, some of the children who received negative speech therapy suffered psychological effects and retained speech problems for the rest of their lives. They were examples of the significance of positive reinforcement in education.
The initial goal of the study was to investigate positive and negative speech therapy. However, the implication spanned much further into methods of teaching for young children.
24. Violinist at the Metro Experiment
Study conducted by: staff at the washington post.
Study Conducted in 2007 at a Washington D.C. Metro Train Station

During the study, pedestrians rushed by without realizing that the musician playing at the entrance to the metro stop was Grammy-winning musician, Joshua Bell. Two days before playing in the subway, he sold out at a theater in Boston where the seats average $100. He played one of the most intricate pieces ever written with a violin worth 3.5 million dollars. In the 45 minutes the musician played his violin, only 6 people stopped and stayed for a while. Around 20 gave him money, but continued to walk their normal pace. He collected $32.
The study and the subsequent article organized by the Washington Post was part of a social experiment looking at:
- the priorities of people
Gene Weingarten wrote about the social experiment: “In a banal setting at an inconvenient time, would beauty transcend?” Later he won a Pulitzer Prize for his story. Some of the questions the article addresses are:
- Do we perceive beauty?
- Do we stop to appreciate it?
- Do we recognize the talent in an unexpected context?
As it turns out, many of us are not nearly as perceptive to our environment as we might like to think.
25. Visual Cliff Experiment
Study conducted by: eleanor gibson and richard walk.
Study Conducted in 1959 at Cornell University
Experiment Details: In 1959, psychologists Eleanor Gibson and Richard Walk set out to study depth perception in infants. They wanted to know if depth perception is a learned behavior or if it is something that we are born with. To study this, Gibson and Walk conducted the visual cliff experiment.
They studied 36 infants between the ages of six and 14 months, all of whom could crawl. The infants were placed one at a time on a visual cliff. A visual cliff was created using a large glass table that was raised about a foot off the floor. Half of the glass table had a checker pattern underneath in order to create the appearance of a ‘shallow side.’
In order to create a ‘deep side,’ a checker pattern was created on the floor; this side is the visual cliff. The placement of the checker pattern on the floor creates the illusion of a sudden drop-off. Researchers placed a foot-wide centerboard between the shallow side and the deep side. Gibson and Walk found the following:
- Nine of the infants did not move off the centerboard.
- All of the 27 infants who did move crossed into the shallow side when their mothers called them from the shallow side.
- Three of the infants crawled off the visual cliff toward their mother when called from the deep side.
- When called from the deep side, the remaining 24 children either crawled to the shallow side or cried because they could not cross the visual cliff and make it to their mother.
What this study helped demonstrate is that depth perception is likely an inborn train in humans.
Among these experiments and psychological tests, we see boundaries pushed and theories taking on a life of their own. It is through the endless stream of psychological experimentation that we can see simple hypotheses become guiding theories for those in this field. The greater field of psychology became a formal field of experimental study in 1879, when Wilhelm Wundt established the first laboratory dedicated solely to psychological research in Leipzig, Germany. Wundt was the first person to refer to himself as a psychologist. Since 1879, psychology has grown into a massive collection of:
- methods of practice
It’s also a specialty area in the field of healthcare. None of this would have been possible without these and many other important psychological experiments that have stood the test of time.
- 20 Most Unethical Experiments in Psychology
- What Careers are in Experimental Psychology?
- 10 Things to Know About the Psychology of Psychotherapy
About Education: Psychology
Explorable.com
Mental Floss.com
About the Author
After earning a Bachelor of Arts in Psychology from Rutgers University and then a Master of Science in Clinical and Forensic Psychology from Drexel University, Kristen began a career as a therapist at two prisons in Philadelphia. At the same time she volunteered as a rape crisis counselor, also in Philadelphia. After a few years in the field she accepted a teaching position at a local college where she currently teaches online psychology courses. Kristen began writing in college and still enjoys her work as a writer, editor, professor and mother.
- 5 Best Online Ph.D. Marriage and Family Counseling Programs
- Top 5 Online Doctorate in Educational Psychology
- 5 Best Online Ph.D. in Industrial and Organizational Psychology Programs
- Top 10 Online Master’s in Forensic Psychology
- 10 Most Affordable Counseling Psychology Online Programs
- 10 Most Affordable Online Industrial Organizational Psychology Programs
- 10 Most Affordable Online Developmental Psychology Online Programs
- 15 Most Affordable Online Sport Psychology Programs
- 10 Most Affordable School Psychology Online Degree Programs
- Top 50 Online Psychology Master’s Degree Programs
- Top 25 Online Master’s in Educational Psychology
- Top 25 Online Master’s in Industrial/Organizational Psychology
- Top 10 Most Affordable Online Master’s in Clinical Psychology Degree Programs
- Top 6 Most Affordable Online PhD/PsyD Programs in Clinical Psychology
- 50 Great Small Colleges for a Bachelor’s in Psychology
- 50 Most Innovative University Psychology Departments
- The 30 Most Influential Cognitive Psychologists Alive Today
- Top 30 Affordable Online Psychology Degree Programs
- 30 Most Influential Neuroscientists
- Top 40 Websites for Psychology Students and Professionals
- Top 30 Psychology Blogs
- 25 Celebrities With Animal Phobias
- Your Phobias Illustrated (Infographic)
- 15 Inspiring TED Talks on Overcoming Challenges
- 10 Fascinating Facts About the Psychology of Color
- 15 Scariest Mental Disorders of All Time
- 15 Things to Know About Mental Disorders in Animals
- 13 Most Deranged Serial Killers of All Time

Site Information
- About Online Psychology Degree Guide
- Random article
- Teaching guide
- Privacy & cookies

by Chris Woodford . Last updated: January 6, 2023.
Photo: There are always new theories to test and experiments to try. Even when we've completely nailed how Earth works, there's still the rest of the Universe to explore! Fourier telescope experiment photo by courtesy of NASA .
1: Galileo demonstrates that objects fall at the same speed (1589)
Photo: Galileo proved that different things fall at the same speed.
2: Isaac Newton splits white light into colors (1672)
Artwork: A glass prism splits white light into a spectrum. Nature recreates Newton's famous experiment whenever you see a rainbow!
3: Henry Cavendish weighs the world (1798)
Artwork: Henry Cavendish's experiment seen from above. 1) Two small balls, connected by a stick, are suspended by a thread so they're free to rotate. 2) The balls are attracted by two much larger (more massive) balls, fixed in place. 3) A light beam shines from the side at a mirror (green), mounted so it moves with the small balls. The beam is reflected back onto a measuring scale. 4) As the two sets of balls attract, the mirror pivots, shifting the reflected beam along the scale, so allowing the movement to be measured.
4: Thomas Young proves light is a wave... or does he? (1803)
Artwork: Thomas Young's famous double-slit experiment proved that light behaved like a wave—at least, some of the time. Left: A laser (1) produces coherent (regular, in-step) light (2) that passes through a pair of slits (3) onto a screen (4). If Newton were completely correct, we'd expect to see a single bright area on the screen and darkness either side. What we actually see is shown on the right. Light appears to ripple out in waves from the two slits (5), producing a distinctive interference pattern of light and dark areas (6).
5: James Prescott Joule demonstrates the conservation of energy (1840)
Artwork: The "Mechanical Equivalent of Heat"—James Prescott Joule's famous experiment proving the law now known as the conservation of energy.
6: Hippolyte Fizeau measures the speed of light (1851)
Artwork: How Fizeau measured the speed of light.
7: Robert Millikan measures the charge on the electron (1909)
Artwork: How Millikan measured the charge on the electron. 1) Oil drops (yellow) are squirted into the experimental apparatus, which has a large positive plate (blue) on top and a large negative plate (red) beneath. 2) X rays (green) are fired in. 3) The X rays give the oil drops a negative electrical charge. 4) The negatively charged drops can be made to "float" in between the two plates so their weight (red) is exactly balanced by the upward electrical pull of the positive plate (blue). When these two forces are equal, we can easily calculate the charge on the drops, which is always a whole number multiple of the basic charge on the electron.
8: Ernest Rutherford (and associates) split the atom (1897–1932)
Artwork: Transmutation: When Rutherford fired alpha particles (helium nuclei) at nitrogen, he produced oxygen. As he later wrote: "We must conclude that the nitrogen atom is disintegrated under the intense forces developed in a close collision with a swift alpha particle, and that the hydrogen atom which is liberated formed a constituent part of the nitrogen nucleus." In other words, he had split one atom apart to make another one.
Artwork: In Rutherford's gold-foil experiment (also known as the Geiger-Marsden experiment), atoms in a sheet of gold foil (1) allow positively charged alpha particles to pass through them (2) as long as the particles are traveling clear of the nucleus. Any particles fired at the nucleus are deflected by its positive charge (3). Fired at exactly the right angle, they will bounce right back! While this experiment is not splitting any atoms, as such, it was a key part of the decades-long effort to understand what atoms are made of—and in that sense, it did help physicists to "split" (venture inside) the atom.
9: Enrico Fermi demonstrates the nuclear chain reaction (1942)
Artwork: The nuclear chain reaction that turns uranium-235 into uranium-236 with a huge release of energy.
10: Rosalind Franklin photographs DNA with X rays (1953)
Artwork: The double-helix structure of DNA. Photographed with X rays, these intertwined curves appear as an X shape. Studying the X pattern in one of Franklin's photos was an important clue that tipped off Crick and Watson about the double helix.
If you liked this article...
Don't want to read our articles try listening instead, find out more, on this website.
- Six Easy Pieces by Richard Feynman. Basic Books, 2011. This book isn't half as "easy" as the title suggests, but it does contain interesting introductions to some of the topics covered here, including the conservation of energy, the double-slit experiment, and quantum theory.
- The Oxford Handbook of the History of Physics by Jed Z. Buchwald and Robert Fox (eds). Oxford University Press, 2013/2017. A collection of twenty nine scholarly essays charting the history of physics from Galileo's gravity to the age of silicon chips.
- Great Experiments in Physics: Firsthand Accounts from Galileo to Einstein Edited by Maurice Shamos. Dover, 1959/1987. This is one of my favorite science books, ever. It's a great compilation of some classic physics experiments (including four of those listed here—the experiments by Henry Cavendish, Thomas Young, James Joule, and Robert Millikan) written by the experimenters themselves. A rare opportunity to read firsthand accounts of first-rate science!
Text copyright © Chris Woodford 2012, 2023. All rights reserved. Full copyright notice and terms of use .
Rate this page
Tell your friends, cite this page, more to explore on our website....
- Get the book
- Send feedback
We have emailed you a PDF version of the article you requested.
Can't find the email?
Please check your spam or junk folder
You can also add [email protected] to your safe senders list to ensure you never miss a message from us.
20 Awesome Science Experiments You Can Do Right Now At Home
Complete the form below and we will email you a PDF version
Cancel and go back
IFLScience needs the contact information you provide to us to contact you about our products and services. You may unsubscribe from these communications at any time.
For information on how to unsubscribe, as well as our privacy practices and commitment to protecting your privacy, check out our Privacy Policy
Complete the form below to listen to the audio version of this article
Advertisement
Subscribe today for our Weekly Newsletter in your inbox!
Morenike Adebayo
Guest Author
DOWNLOAD PDF VERSION

We can all agree that science is awesome. And you can bring that awesomeness into your very own home with these 20 safe DIY experiments you can do right now with ordinary household items.
1. Make Objects Seemingly Disappear Refraction is when light changes direction and speed as it passes from one object to another. Only visible objects reflect light. When two materials with similar reflective properties come into contact, light will pass through both materials at the same speed, rendering the other material invisible. Check out this video from BritLab on how to turn glass invisible using vegetable oil and pyrex glass.
2. Freeze Water Instantly When purified water is cooled to just below freezing point, a quick nudge or an icecube placed in it is all it takes for the water to instantly freeze. You can finally have the power of Frozone from The Incredibles on a very small scale! Check out the video on this "cool" experiment.
3. Create Oobleck And Make It Dance To The Music Named after a sticky substance in a children’s book by Dr Seuss , Oobleck is a non-Newtonian fluid, which means it can behave as both a solid and a liquid. And when placed on a sound source, the vibrations causes the mixture to gloopily dance. Check out these instructions from Housing A Forest on how to make this groovy fluid funk out in every way.
4. Create Your Own Hybrid Rocket Engine With a combination of a solid fuel source and a liquid oxidizer, hybrid rocket engines can propel themselves. And on a small scale, you can create your own hybrid rocket engine, using pasta, mouthwash and yeast. Sadly, it won’t propel much, but who said rocket science ain’t easy? Check out this video from NightHawkInLight on how to make this mini engine.
5. Create "Magic Mud" Another non-Newtonian fluid here, this time from the humble potato. "Magic Mud" is actually starch found in potatoes. It’ll remain hard when handled but leave it alone and it turns into a liquid. Make your own “Magic Mud” with this video.
6. Command The Skies And Create A Cloud In A Bottle Not quite a storm in a teacup, but it is a cloud in a bottle. Clouds up in the sky are formed when water vapor cools and condenses into visible water droplets. Create your own cloud in a bottle using a few household items with these wikiHow instructions .
7. Create An Underwater Magical World First synthesized by Adolf van Baeyer in 1871, fluorescein is a non-toxic powder found in highlighter pens, and used by NASA to find shuttles that land in the sea. Create an underwater magical world with this video from NightHawkInLight .
9. Make Your Own Lava Lamp Inside a lava lamp are colored bubbles of wax suspended in a clear or colorless liquid, which changes density when warmed by a heating element at the base, allowing them to rise and fall hypnotically. Create your own lava lamp with these video instructions.
10. Create Magnetic Fluid A ferrofluid is a liquid that contains nanoscale particles of metal, which can become magnetized. And with oil, toner and a magnet , you can create your own ferrofluid and harness the power of magnetism!
12. Make Waterproof Sand A hydrophobic substance is one that repels water. When sand is combined with a water-resistant chemical, it becomes hydrophobic. So when it comes into contact with water, the sand will remain dry and reusable. Make your own waterproof sand with this video .
13. Make Elephant's Toothpaste Elephant’s toothpaste is a steaming foamy substance created by the rapid decomposition of hydrogen peroxide, which sort of resembles giant-sized toothpaste. Make your own elephant’s toothpaste with these instructions.
14. Make Crystal Bubbles When the temperature falls below 0 o C (32 o F), it’s possible to freeze bubbles into crystals. No instructions needed here, just some bubble mix and chilly weather.
15. Make Moving Liquid Art Mixing dish soap and milk together causes the surface tension of the milk to break down. Throw in different food colorings and create this trippy chemical reaction.
16. Create Colourful Carnations Flowers absorb water through their stems, and if that water has food coloring in it, the flowers will also absorb that color. Create some wonderfully colored flowers with these wikiHow instructions .
17. "Magically" Turn Water Into Wine Turn water into wine with this video by experimenter Dave Hax . Because water has a higher density than wine, they can switch places. Amaze your friends with this fun science trick.
18. Release The Energy In Candy (Without Eating It) Dropping a gummy bear into a test tube with potassium chlorate releases the chemical energy inside in an intense chemical reaction. That’s exactly what's happening when you eat candy, kids.
19. Make Water "Mysteriously" Disappear Sodium polyacrylate is a super-absorbent polymer, capable of absorbing up to 300 times its own weight in water. Found in disposable diapers, you can make water disappear in seconds with this video .
20. Create A Rainbow In A Jar Different liquids have different masses and different densities. For example, oil is less dense than water and will float on top of its surface. By combining liquids of different densities and adding food coloring, you can make an entire rainbow in a jar with this video .
There you have it – 20 experiments for you to explore the incredible world of science!
ARTICLE POSTED IN
experiment,
fluorescein,
rocket engine,
hydrogen peroxide,
sodium acetate,
ferrofluid,
More Space and Physics Stories
link to article

JWST Sees Hydrogen Emission Line From Time When The Universe Should Have Been Opaque
![10 great experiments An artist’s concept looks down into the core of the galaxy M87, which is just left of centre and appears as a large blue dot. A bright blue-white, narrow and linear jet of plasma transects the illustration from centre left to upper right. It begins at the source of the jet, the galaxy’s black hole, which is surrounded by a blue spiral of material. At lower right is a red giant star that is far from the black hole and close to the viewer. A bridge of glowing gas links the star to a smaller white dwarf star companion immediately to its left. Engorged with infalling hydrogen from the red giant star, the smaller star exploded in a blue-white flash, which looks like numerous diffraction spikes emitted in all directions. Thousands of stars are in the background.]](https://assets.iflscience.com/assets/articleNo/76155/aImg/79193/jet-m.jpg)
Powerful Black Hole Jets Might Be Triggering Unrelated Nova Explosions

Site of Famous Arecibo Telescope Seeks To Move From Astronomy To Education

Biblical Seeds, World’s Oldest Cheese, And A Fish With Tongues For Legs

IFLScience We Have Questions: How Do Sunken Cities End Up Underwater?

Gorilla Dicks, Life After Death, And Earth's New (Mini) Moon
10 Cool Chemistry Experiments
ThoughtCo / Hilary Allison
- Projects & Experiments
- Chemical Laws
- Periodic Table
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Chemistry is king when it comes to making science cool. There are many interesting and fun projects to try, but these 10 chemistry experiments might be the coolest.
Whether you want to witness color transformations with copper and nitric acid or create a foam spectacle with hydrogen peroxide and potassium iodide, there's something here to spark curiosity in everyone. There's even a famous chemical reaction that will emit blue light and a characteristic barking or woofing sound.
Copper and Nitric Acid
When you place a piece of copper in nitric acid , the Cu 2+ ions and nitrate ions coordinate to color the solution green and then brownish-green. If you dilute the solution, water displaces nitrate ions around the copper, and the solution changes to blue.
Hydrogen Peroxide with Potassium Iodide
Affectionately known as elephant toothpaste , the chemical reaction between peroxide and potassium iodide shoots out a column of foam. If you add food coloring, you can customize the "toothpaste" for holiday-colored themes.
Any Alkali Metal in Water
Any of the alkali metals will react vigorously in water . How vigorously? Sodium burns bright yellow. Potassium burns violet. Lithium burns red. Cesium explodes. Experiment by moving down the alkali metals group of the periodic table.
Thermite Reaction
The thermite reaction essentially shows what would happen if iron rusted instantly, rather than over time. In other words, it's making metal burn. If the conditions are right, just about any metal will burn. However, the reaction usually is performed by reacting iron oxide with aluminum:
Fe 2 O 3 + 2Al → 2Fe + Al 2 O 3 + heat and light
If you want a truly stunning display, try placing the mixture inside a block of dry ice and then lighting the mixture.
Coloring Fire
SEAN GLADWELL / Getty Images
When ions are heated in a flame, electrons become excited and then drop to a lower energy state, emitting photons. The energy of the photons is characteristic of the chemical and corresponds to specific flame colors . It's the basis for the flame test in analytical chemistry , plus it's fun to experiment with different chemicals to see what colors they produce in a fire.
Make Polymer Bouncy Balls
Who doesn't enjoy playing with bouncy balls ? The chemical reaction used to make the balls makes a terrific experiment because you can alter the properties of the balls by changing the ratio of the ingredients.
Make a Lichtenberg Figure
A Lichtenberg figure or "electrical tree" is a record of the path taken by electrons during an electrostatic discharge. It's basically frozen lightning. There are several ways you can make an electrical tree.
Experiment with 'Hot Ice'
Hot ice is a name given to sodium acetate, a chemical you can make by reacting vinegar and baking soda. A solution of sodium acetate can be supercooled so that it will crystallize on command. Heat is evolved when the crystals form, so although it resembles water ice, it's hot.
Barking Dog Experiment
The Barking Dog is the name given to a chemiluminescent reaction involving the exothermic combination of either nitrous oxide or nitrogen monoxide with carbon disulfide. The reaction proceeds down a tube, emitting blue light and a characteristic "woof" sound.
Another version of the demonstration involves coating the inside of a clear jug with alcohol and igniting the vapor. The flame front proceeds down the bottle , which also barks.
Dehydration of Sugar
When you react sugar with sulfuric acid , the sugar is violently dehydrated. The result is a growing column of carbon black, heat, and the overwhelming odor of burnt caramel.
Easy Science Experiments
Want something less extravagant but still fun? These easy science experiments are doable with items you likely already have at home—from creating invisible ink with baking soda to making homemade ice cream in a plastic bag.
- Where to Buy Saltpeter or Potassium Nitrate
- Vitamin C Determination by Iodine Titration
- Cool Dry Ice Experiments
- How to Make Bubbles That Don't Pop
- Equation for the Reaction Between Baking Soda and Vinegar
- 5 Ways to Make Glue
- How to Grow Table Salt or Sodium Chloride Crystals
- Color Change Chemistry Experiments
- How to Melt Aluminum Cans at Home
- 10 Cool Chemistry Demonstrations for Educators
- Science Projects for Every Subject
- 10 Fun Chemistry Demonstrations and Experiments
- Valentine's Day Chemistry
- Thames & Kosmos Chem 3000 Chemistry Kit Review
- Halloween Reaction or Old Nassau Reaction
- How to Do the Color Change Chameleon Chemistry Demonstration
Cool Science Experiments Headquarters
Making Science Fun, Easy to Teach and Exciting to Learn!
Science Experiments
35 Easy Science Experiments You Can Do Today!
Looking for easy science experiments to do at home or in the classroom? You’re in luck because we’ve got over 35 easy science activities for kids that will help you make science fun for all ages.
Most of these simple science experiments for kids are easy to prepare, quick to perform, and use household items or inexpensive materials you can find almost anywhere. To connect the fun to the “why it works” you’ll find an easy to teach explanation with every experiment!
Musical Jars Science Experiment

This super easy experiment is simple as it is fun! Kids make their own musical instruments with clear jars and water then investigate sound waves, pitch, and more.
When the experiment is complete, use the colorful new “instrument” for a fun music lesson. Kids can play and take turns to “name that tune”!
Detailed Instructions & Video Tutorial -> Musical Jars Science Experiment
Viscosity of Liquids Science Experiment

Viscosity may be a confusing term for kids at first, but this super easy experiment can help them see viscosity in action!
With marbles, clear jars, and a few household materials, kids will make predictions, record data, and compare the results while they test high and low density liquids.
Detailed Instructions & Video Tutorial -> Viscosity Science Experiment
Floating Egg Science Experiment

Can a solid egg float? Kids can find the answer and understand why with this quick science experiment.
Discover just how easy it can be to make a raw egg float while testing the laws of density. We’ve included additional ideas to try so kids can make predictions and test the concept further.
Detailed Instructions & Video Tutorial -> Floating Egg Science Experiment
Paper Towel Dry Under Water Experiment

Is it possible to keep a paper towel dry even when submerging it under water? The answer is a surprising “yes,” if you use science to help!
Start with the properties of your materials, make a prediction, then explore matter, density, volume, and more.
Detailed Instructions & Video Tutorial -> Paper Towel Dry Under Water Experiment
Mixing Oil & Water Science Experiment

This simple experiment for kids helps them better understand density and the changes that happen when adding an emulsifier to the mix.
Detailed Instructions & Video Tutorial -> Mixing Oil & Water Experiment
Will it Float or Sink Science Experiment
Will it sink or will it float? This fun experiment challenges what students think they know about household items!
Students record their hypothesis for each item then test it to compare what they think will happen against their observations.
Detailed Instructions & Video Tutorial -> Float or Sink Science Experiment
Water Temperature Science Experiment

What does thermal energy look like? In this easy science experiment, kids are able to see thermal energy as they explore the concept in action.
With clear jars and food coloring, students can quickly see how molecules move differently through hot and cold water.
Detailed Instructions & Video Tutorial -> Water Temperature Science Experiment
Balloon Blow-up Science Experiment

Kids will discover how matter reacts when heated and cooled as they watch with surprise as baking soda and vinegar blow the balloon up before their eyes.
Detailed Instructions & Video Tutorial -> Balloon Blow-up Science Experiment
Floating Ping Pong Ball Science Experiment

Kids will giggle with joy with this super easy experiment. With only a ping pong ball and a hair dryer, students will have a great time while exploring Bernoulli’s Principle in action.
We’ve included additional ideas to further explore the concept with different objects and observe the change in results.
Detailed Instructions & Video Tutorial -> Floating Ping Pong Ball Science Experiment
Hair Stand on End Science Experiment

It’s especially fun for those who’ve never seen static electricity in action before!
Detailed Instructions & Video Tutorial -> Hair Stand on End Science Experiment
Oil Bubbles in Water Science Experiment
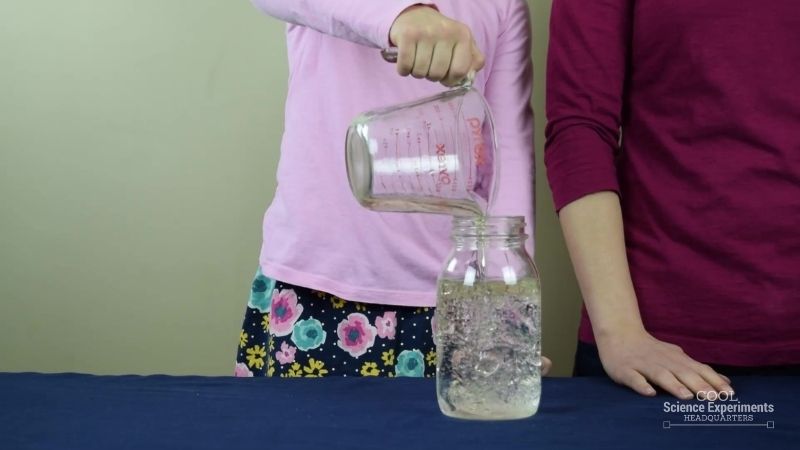
Kids explore density and experience some chemistry when creating oil bubbles in water with everyday household items.
This experiment is particularly fun when kids see that they’ve made what looks like a lava lamp!
Detailed Instructions & Video Tutorial -> Oil Bubbles in Water Science Experiment
Color Changing Water Science Experiment

Kids will be surprised as they watch a new color being “created” without mixing! Using only a clear bowl and glass, some food coloring, and water, this super easy science experiment is quick and easy with a huge wow factor.
Try it with yellow and blue to follow along with our demonstration video then try different primary color combinations and explore the results.
Detailed Instructions & Video Tutorial -> Color Changing Water Science Experiment
Magnetic Paper Clip Chain Science Experiment

It may seem a bit like magic but it’s actually science! It’s not hard to capture your kids’ attention with this quick and easy science experiment as they watch paper clips “stick” together and form a chain!
Perfect for younger children, the experiment only takes a few minutes and is a fun way to explore the concept of magnetic transference.
Detailed Instructions & Video Tutorial -> Magnetic Paper Clip Chain Science Experiment
Is it Magnetic Science Experiment
With only a magnet and a few household items, kids will make and record their predictions, test and observe, then compare what they think is magnetic against the results.
Simple and quick, but some of the results may surprise your students!
Cloud in a Jar Experiment

This simple experiment only requires a few materials but really holds student attention as a cloud forms before their eyes!
Kids will learn new weather vocabulary as they explore how physical changes and reactions happen as clouds begin to take form. We’ve also included a helpful chart on the types of clouds.
Detailed Instructions & Video Tutorial -> Cloud in a Jar Science Experiment
Magic Milk Science Experiment

Create a dancing rainbow of colors with this easy science experiment for kids!
Using only a few ordinary kitchen items, your students can create a color explosion in ordinary milk when they add our special ingredient. (Hint: The special ingredient (soap!) includes hydrophilic and hydrophobic molecules that make the magic happen!)
Detailed Instructions & Video Tutorial -> Magic Milk Science Experiment
Walking Water Science Experiment

Water can’t really walk upwards against gravity, but this cool science experiment makes it seem like it can!
Kids are able to see the capillary action process and learn how attraction and adhesive forces in action allow water to move out of one glass into another.
Detailed Instructions & Video Tutorial -> Walking Water Science Experiment
Light Refraction Science Experiment
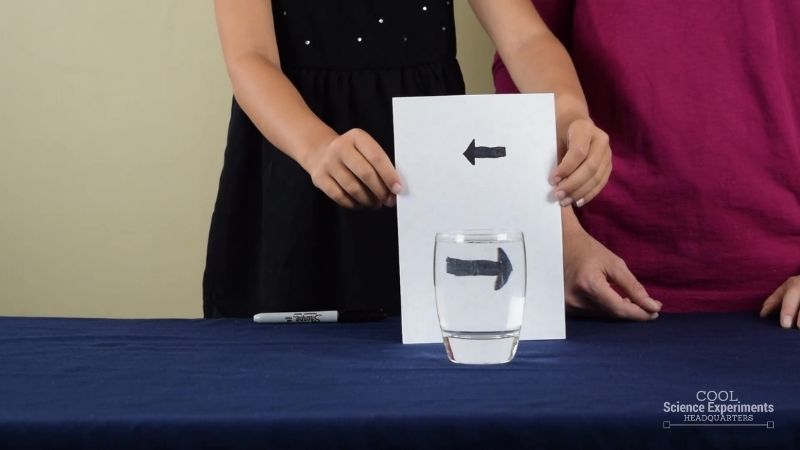
The results of this easy science experiment are so amazing, it makes kids (and adults) think it must be magic!
Young scientists watch in surprise while they see an arrow change directions instantly. Investigating refraction couldn’t be more fun!
Detailed Instructions & Video Tutorial -> Light Refraction Science Experiment
Dancing Raisins Experiment

Learn about the reactions of buoyancy and density in this simple science activity for kids.
They may not need dancing shoes, but give them a glass of soda pop and the raisins in this fun experiment love to dance!
Detailed Instructions & Video Tutorial -> Dancing Raisins Science Experiment
See Sound Experiment

Kids love this experiment because they are encouraged to drum loudly so they can “see” sound waves in action!
Detailed Instructions & Video Tutorial -> See Sound Science Experiment
Elephant Toothpaste Science Experiment

Grab some giant brushes and get ready to make elephant toothpaste! Although you might not be able to get an elephant excited by this super easy experiment, kids love it!
The impressive and quick results created by the chemical reaction and the heat released in the process makes an abundant amount of fun and colorful foam!
Detailed Instructions & Video Tutorial -> Elephant Toothpaste Science Experiment
Upside Down Glass of Water Science Experiment

We all know what happens when we turn a glass of water upside down, but what if I told you you can do it without the water spilling out?
The experiment only requires a few common items and you’ll be amazed by the results of air pressure in action!
Detailed Instructions & Video Tutorial -> Upside Down Glass of Water Science Experiment
Pick up Ball with a Jar Science Experiment

It almost seems like magic but with the help of science, you can pick up a ball with an open jar!
Instead of magic, this easy science activity uses centripetal force and practice to do what seems like the impossible.
Detailed Instructions & Video Tutorial -> Pick up Ball with a Jar Experiment
Will It Melt Science Experiment
Can you guess which items will melt? This easy outside experiment challenges what students think they know about the effects of the sun.
Pepper Move Science Experiment

Can you make pepper move and zoom away with just a light touch of your finger? With science you can!
This experiment only takes a few quick minutes from beginning to end, but the reaction caused by surface tension makes kids want to do it over and over.
Detailed Instructions & Video Tutorial -> Pepper Move Science Experiment
Crush a Plastic Bottle Science Experiment

Go for it, crush that bottle, but don’t touch it! Although it usually can’t be seen or touched, air pressure is pushing against all surfaces at all times.
With this easy science activity kids can see air pressure at work when they watch a bottle crushes itself!
Detailed Instructions & Video Tutorial -> Crush a Plastic Bottle Science Experiment
Egg in Vinegar Science Experiment

This vinegar science experiment will have your eggs and kids bouncing (with excitement!) before you know it!
Kids can watch and explore the results of chemical reactions as the egg changes from something that seems solid into what feels like something bouncy!
Detailed Instructions & Video Tutorial -> Egg in Vinegar Science Experiment
Straw Through a Potato Science Experiment

Can you make a normal plastic straw go into a raw, solid potato? It seems like something impossible, but science can easily make it possible!
Pick your potatoes then let kids try their strength as they explore air pressure with this super easy experiment.
Detailed Instructions & Video Tutorial -> Straw Through a Potato Science Experiment
Rainbow in a Jar Science Experiment

With only a few household items, they’ll explore mass, volume, and density with every color layer!
Detailed Instructions & Video Tutorial -> Rainbow in a Jar Experiment
Tornado in a Bottle Science Experiment

Kids can have fun while learning more about centripetal force with this fun experiment.
With a little muscle and science, kids watch with amazement as they create their own glitter cyclone in a bottle as the centripetal force vortex appears.
Detailed Instructions & Video Tutorial -> Tornado in a Bottle Science Experiment
Why Doesn’t the Water Leak Science Experiment

Can you poke holes in a plastic bag full of water without the water leaking out? With this super easy science activity you can!
Kids are stunned as they learn about polymers and how they can do what seems to be impossible.
Detailed Instructions & Video Tutorial -> Why Doesn’t the Water Leak Science Experiment
Use a Bottle to Blow-up a Balloon Experiment

Is it possible to blow up a balloon with only water and science?
In this super easy experiment, kids learn more about how matter behaves as they watch a balloon inflate and deflate as a result of matter being heated and cooled.
Detailed Instructions & Video Tutorial -> Use a Bottle to Blow-up a Balloon Experiment
Orange Float Science Experiment

Kids explore buoyancy as they learn about and test density in this sink or float science activity.
While it only takes a few minutes, this super easy experiment invites kids to predict what they think will happen then discuss why the heavier orange floats!
Detailed Instructions & Video Tutorial -> Orange Float Science Experiment
Pick up Ice with String Science Experiment

With only a few household items, kids learn about freezing temperatures and the results they create in saltwater versus freshwater.
Detailed Instructions & Video Tutorial -> Pick Up Ice with String Science Experiment
Color Changing Walking Water Experiment

Using the concepts explored in our popular Walking Water Science Experiment, kids will see color walk from one glass to another and change colors as it goes!
The quick experiment seems to defy gravity like magic, but don’t worry, kids can find out how science makes it work!
Detailed Instructions & Video Tutorial -> Color Changing Walking Water Experiment
Reader Interactions
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

- Privacy Policy
- Disclosure Policy
Copyright © 2024 · Cool Science Experiments HQ
- Grades 6-12
- School Leaders
Get our mega Halloween worksheets bundle! 👻
72 Easy Science Experiments Using Materials You Already Have On Hand
Because science doesn’t have to be complicated.

If there is one thing that is guaranteed to get your students excited, it’s a good science experiment! While some experiments require expensive lab equipment or dangerous chemicals, there are plenty of cool projects you can do with regular household items. We’ve rounded up a big collection of easy science experiments that anybody can try, and kids are going to love them!
Easy Chemistry Science Experiments
Easy physics science experiments, easy biology and environmental science experiments, easy engineering experiments and stem challenges.

1. Taste the Rainbow
Teach your students about diffusion while creating a beautiful and tasty rainbow! Tip: Have extra Skittles on hand so your class can eat a few!
Learn more: Skittles Diffusion

2. Crystallize sweet treats
Crystal science experiments teach kids about supersaturated solutions. This one is easy to do at home, and the results are absolutely delicious!
Learn more: Candy Crystals
3. Make a volcano erupt
This classic experiment demonstrates a chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid), which produces carbon dioxide gas, water, and sodium acetate.
Learn more: Best Volcano Experiments
4. Make elephant toothpaste
This fun project uses yeast and a hydrogen peroxide solution to create overflowing “elephant toothpaste.” Tip: Add an extra fun layer by having kids create toothpaste wrappers for plastic bottles.
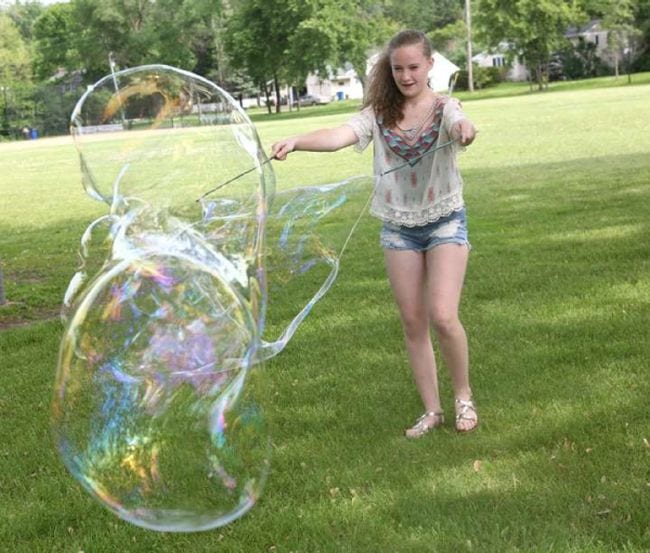
5. Blow the biggest bubbles you can
Add a few simple ingredients to dish soap solution to create the largest bubbles you’ve ever seen! Kids learn about surface tension as they engineer these bubble-blowing wands.
Learn more: Giant Soap Bubbles

6. Demonstrate the “magic” leakproof bag
All you need is a zip-top plastic bag, sharp pencils, and water to blow your kids’ minds. Once they’re suitably impressed, teach them how the “trick” works by explaining the chemistry of polymers.
Learn more: Leakproof Bag

7. Use apple slices to learn about oxidation
Have students make predictions about what will happen to apple slices when immersed in different liquids, then put those predictions to the test. Have them record their observations.
Learn more: Apple Oxidation
8. Float a marker man
Their eyes will pop out of their heads when you “levitate” a stick figure right off the table! This experiment works due to the insolubility of dry-erase marker ink in water, combined with the lighter density of the ink.
Learn more: Floating Marker Man

9. Discover density with hot and cold water
There are a lot of easy science experiments you can do with density. This one is extremely simple, involving only hot and cold water and food coloring, but the visuals make it appealing and fun.
Learn more: Layered Water

10. Layer more liquids
This density demo is a little more complicated, but the effects are spectacular. Slowly layer liquids like honey, dish soap, water, and rubbing alcohol in a glass. Kids will be amazed when the liquids float one on top of the other like magic (except it is really science).
Learn more: Layered Liquids

11. Grow a carbon sugar snake
Easy science experiments can still have impressive results! This eye-popping chemical reaction demonstration only requires simple supplies like sugar, baking soda, and sand.
Learn more: Carbon Sugar Snake
12. Mix up some slime
Tell kids you’re going to make slime at home, and watch their eyes light up! There are a variety of ways to make slime, so try a few different recipes to find the one you like best.

13. Make homemade bouncy balls
These homemade bouncy balls are easy to make since all you need is glue, food coloring, borax powder, cornstarch, and warm water. You’ll want to store them inside a container like a plastic egg because they will flatten out over time.
Learn more: Make Your Own Bouncy Balls

14. Create eggshell chalk
Eggshells contain calcium, the same material that makes chalk. Grind them up and mix them with flour, water, and food coloring to make your very own sidewalk chalk.
Learn more: Eggshell Chalk

15. Make naked eggs
This is so cool! Use vinegar to dissolve the calcium carbonate in an eggshell to discover the membrane underneath that holds the egg together. Then, use the “naked” egg for another easy science experiment that demonstrates osmosis .
Learn more: Naked Egg Experiment
16. Turn milk into plastic
This sounds a lot more complicated than it is, but don’t be afraid to give it a try. Use simple kitchen supplies to create plastic polymers from plain old milk. Sculpt them into cool shapes when you’re done!

17. Test pH using cabbage
Teach kids about acids and bases without needing pH test strips! Simply boil some red cabbage and use the resulting water to test various substances—acids turn red and bases turn green.
Learn more: Cabbage pH

18. Clean some old coins
Use common household items to make old oxidized coins clean and shiny again in this simple chemistry experiment. Ask kids to predict (hypothesize) which will work best, then expand the learning by doing some research to explain the results.
Learn more: Cleaning Coins

19. Pull an egg into a bottle
This classic easy science experiment never fails to delight. Use the power of air pressure to suck a hard-boiled egg into a jar, no hands required.
Learn more: Egg in a Bottle
20. Blow up a balloon (without blowing)
Chances are good you probably did easy science experiments like this when you were in school. The baking soda and vinegar balloon experiment demonstrates the reactions between acids and bases when you fill a bottle with vinegar and a balloon with baking soda.
21 Assemble a DIY lava lamp
This 1970s trend is back—as an easy science experiment! This activity combines acid-base reactions with density for a totally groovy result.

22. Explore how sugary drinks affect teeth
The calcium content of eggshells makes them a great stand-in for teeth. Use eggs to explore how soda and juice can stain teeth and wear down the enamel. Expand your learning by trying different toothpaste-and-toothbrush combinations to see how effective they are.
Learn more: Sugar and Teeth Experiment
23. Mummify a hot dog
If your kids are fascinated by the Egyptians, they’ll love learning to mummify a hot dog! No need for canopic jars , just grab some baking soda and get started.
24. Extinguish flames with carbon dioxide
This is a fiery twist on acid-base experiments. Light a candle and talk about what fire needs in order to survive. Then, create an acid-base reaction and “pour” the carbon dioxide to extinguish the flame. The CO2 gas acts like a liquid, suffocating the fire.

25. Send secret messages with invisible ink
Turn your kids into secret agents! Write messages with a paintbrush dipped in lemon juice, then hold the paper over a heat source and watch the invisible become visible as oxidation goes to work.
Learn more: Invisible Ink
26. Create dancing popcorn
This is a fun version of the classic baking soda and vinegar experiment, perfect for the younger crowd. The bubbly mixture causes popcorn to dance around in the water.

27. Shoot a soda geyser sky-high
You’ve always wondered if this really works, so it’s time to find out for yourself! Kids will marvel at the chemical reaction that sends diet soda shooting high in the air when Mentos are added.
Learn more: Soda Explosion

28. Send a teabag flying
Hot air rises, and this experiment can prove it! You’ll want to supervise kids with fire, of course. For more safety, try this one outside.
Learn more: Flying Tea Bags

29. Create magic milk
This fun and easy science experiment demonstrates principles related to surface tension, molecular interactions, and fluid dynamics.
Learn more: Magic Milk Experiment
30. Watch the water rise
Learn about Charles’s Law with this simple experiment. As the candle burns, using up oxygen and heating the air in the glass, the water rises as if by magic.
Learn more: Rising Water

31. Learn about capillary action
Kids will be amazed as they watch the colored water move from glass to glass, and you’ll love the easy and inexpensive setup. Gather some water, paper towels, and food coloring to teach the scientific magic of capillary action.
Learn more: Capillary Action

32. Give a balloon a beard
Equally educational and fun, this experiment will teach kids about static electricity using everyday materials. Kids will undoubtedly get a kick out of creating beards on their balloon person!
Learn more: Static Electricity
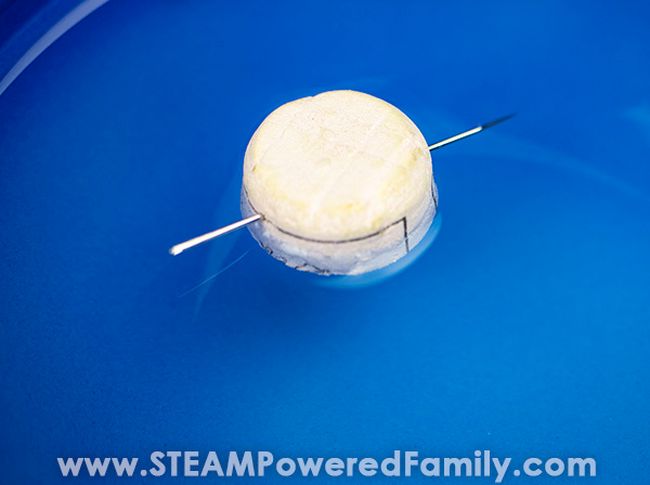
33. Find your way with a DIY compass
Here’s an old classic that never fails to impress. Magnetize a needle, float it on the water’s surface, and it will always point north.
Learn more: DIY Compass
34. Crush a can using air pressure
Sure, it’s easy to crush a soda can with your bare hands, but what if you could do it without touching it at all? That’s the power of air pressure!

35. Tell time using the sun
While people use clocks or even phones to tell time today, there was a time when a sundial was the best means to do that. Kids will certainly get a kick out of creating their own sundials using everyday materials like cardboard and pencils.
Learn more: Make Your Own Sundial
36. Launch a balloon rocket
Grab balloons, string, straws, and tape, and launch rockets to learn about the laws of motion.

37. Make sparks with steel wool
All you need is steel wool and a 9-volt battery to perform this science demo that’s bound to make their eyes light up! Kids learn about chain reactions, chemical changes, and more.
Learn more: Steel Wool Electricity
38. Levitate a Ping-Pong ball
Kids will get a kick out of this experiment, which is really all about Bernoulli’s principle. You only need plastic bottles, bendy straws, and Ping-Pong balls to make the science magic happen.

39. Whip up a tornado in a bottle
There are plenty of versions of this classic experiment out there, but we love this one because it sparkles! Kids learn about a vortex and what it takes to create one.
Learn more: Tornado in a Bottle

40. Monitor air pressure with a DIY barometer
This simple but effective DIY science project teaches kids about air pressure and meteorology. They’ll have fun tracking and predicting the weather with their very own barometer.
Learn more: DIY Barometer

41. Peer through an ice magnifying glass
Students will certainly get a thrill out of seeing how an everyday object like a piece of ice can be used as a magnifying glass. Be sure to use purified or distilled water since tap water will have impurities in it that will cause distortion.
Learn more: Ice Magnifying Glass

42. String up some sticky ice
Can you lift an ice cube using just a piece of string? This quick experiment teaches you how. Use a little salt to melt the ice and then refreeze the ice with the string attached.
Learn more: Sticky Ice
43. “Flip” a drawing with water
Light refraction causes some really cool effects, and there are multiple easy science experiments you can do with it. This one uses refraction to “flip” a drawing; you can also try the famous “disappearing penny” trick .
Learn more: Light Refraction With Water
44. Color some flowers
We love how simple this project is to re-create since all you’ll need are some white carnations, food coloring, glasses, and water. The end result is just so beautiful!

45. Use glitter to fight germs
Everyone knows that glitter is just like germs—it gets everywhere and is so hard to get rid of! Use that to your advantage and show kids how soap fights glitter and germs.
Learn more: Glitter Germs

46. Re-create the water cycle in a bag
You can do so many easy science experiments with a simple zip-top bag. Fill one partway with water and set it on a sunny windowsill to see how the water evaporates up and eventually “rains” down.
Learn more: Water Cycle

47. Learn about plant transpiration
Your backyard is a terrific place for easy science experiments. Grab a plastic bag and rubber band to learn how plants get rid of excess water they don’t need, a process known as transpiration.
Learn more: Plant Transpiration

48. Clean up an oil spill
Before conducting this experiment, teach your students about engineers who solve environmental problems like oil spills. Then, have your students use provided materials to clean the oil spill from their oceans.
Learn more: Oil Spill

49. Construct a pair of model lungs
Kids get a better understanding of the respiratory system when they build model lungs using a plastic water bottle and some balloons. You can modify the experiment to demonstrate the effects of smoking too.
Learn more: Model Lungs

50. Experiment with limestone rocks
Kids love to collect rocks, and there are plenty of easy science experiments you can do with them. In this one, pour vinegar over a rock to see if it bubbles. If it does, you’ve found limestone!
Learn more: Limestone Experiments

51. Turn a bottle into a rain gauge
All you need is a plastic bottle, a ruler, and a permanent marker to make your own rain gauge. Monitor your measurements and see how they stack up against meteorology reports in your area.
Learn more: DIY Rain Gauge

52. Build up towel mountains
This clever demonstration helps kids understand how some landforms are created. Use layers of towels to represent rock layers and boxes for continents. Then pu-u-u-sh and see what happens!
Learn more: Towel Mountains

53. Take a play dough core sample
Learn about the layers of the earth by building them out of Play-Doh, then take a core sample with a straw. ( Love Play-Doh? Get more learning ideas here. )
Learn more: Play Dough Core Sampling

54. Project the stars on your ceiling
Use the video lesson in the link below to learn why stars are only visible at night. Then create a DIY star projector to explore the concept hands-on.
Learn more: DIY Star Projector

55. Make it rain
Use shaving cream and food coloring to simulate clouds and rain. This is an easy science experiment little ones will beg to do over and over.
Learn more: Shaving Cream Rain
56. Blow up your fingerprint
This is such a cool (and easy!) way to look at fingerprint patterns. Inflate a balloon a bit, use some ink to put a fingerprint on it, then blow it up big to see your fingerprint in detail.

57. Snack on a DNA model
Twizzlers, gumdrops, and a few toothpicks are all you need to make this super-fun (and yummy!) DNA model.
Learn more: Edible DNA Model
58. Dissect a flower
Take a nature walk and find a flower or two. Then bring them home and take them apart to discover all the different parts of flowers.

59. Craft smartphone speakers
No Bluetooth speaker? No problem! Put together your own from paper cups and toilet paper tubes.
Learn more: Smartphone Speakers

60. Race a balloon-powered car
Kids will be amazed when they learn they can put together this awesome racer using cardboard and bottle-cap wheels. The balloon-powered “engine” is so much fun too.
Learn more: Balloon-Powered Car

61. Build a Ferris wheel
You’ve probably ridden on a Ferris wheel, but can you build one? Stock up on wood craft sticks and find out! Play around with different designs to see which one works best.
Learn more: Craft Stick Ferris Wheel
62. Design a phone stand
There are lots of ways to craft a DIY phone stand, which makes this a perfect creative-thinking STEM challenge.
63. Conduct an egg drop
Put all their engineering skills to the test with an egg drop! Challenge kids to build a container from stuff they find around the house that will protect an egg from a long fall (this is especially fun to do from upper-story windows).
Learn more: Egg Drop Challenge Ideas

64. Engineer a drinking-straw roller coaster
STEM challenges are always a hit with kids. We love this one, which only requires basic supplies like drinking straws.
Learn more: Straw Roller Coaster

65. Build a solar oven
Explore the power of the sun when you build your own solar ovens and use them to cook some yummy treats. This experiment takes a little more time and effort, but the results are always impressive. The link below has complete instructions.
Learn more: Solar Oven
66. Build a Da Vinci bridge
There are plenty of bridge-building experiments out there, but this one is unique. It’s inspired by Leonardo da Vinci’s 500-year-old self-supporting wooden bridge. Learn how to build it at the link, and expand your learning by exploring more about Da Vinci himself.
Learn more: Da Vinci Bridge
67. Step through an index card
This is one easy science experiment that never fails to astonish. With carefully placed scissor cuts on an index card, you can make a loop large enough to fit a (small) human body through! Kids will be wowed as they learn about surface area.

68. Stand on a pile of paper cups
Combine physics and engineering and challenge kids to create a paper cup structure that can support their weight. This is a cool project for aspiring architects.
Learn more: Paper Cup Stack

69. Test out parachutes
Gather a variety of materials (try tissues, handkerchiefs, plastic bags, etc.) and see which ones make the best parachutes. You can also find out how they’re affected by windy days or find out which ones work in the rain.
Learn more: Parachute Drop

70. Recycle newspapers into an engineering challenge
It’s amazing how a stack of newspapers can spark such creative engineering. Challenge kids to build a tower, support a book, or even build a chair using only newspaper and tape!
Learn more: Newspaper STEM Challenge

71. Use rubber bands to sound out acoustics
Explore the ways that sound waves are affected by what’s around them using a simple rubber band “guitar.” (Kids absolutely love playing with these!)
Learn more: Rubber Band Guitar

72. Assemble a better umbrella
Challenge students to engineer the best possible umbrella from various household supplies. Encourage them to plan, draw blueprints, and test their creations using the scientific method.
Learn more: Umbrella STEM Challenge
Plus, sign up for our newsletters to get all the latest learning ideas straight to your inbox.

You Might Also Like

Magic Milk Experiment: How-To Plus Free Worksheet
This classic experiment teaches kids about basic chemistry and physics. Continue Reading
Copyright © 2024. All rights reserved. 5335 Gate Parkway, Jacksonville, FL 32256
- The Magazine
- Stay Curious
- The Sciences
- Environment
- Planet Earth
10 Scientific Discoveries That Changed The World
Dna, gravity, and germ theory are a few of the key findings in history that forever shifted the course of human civilization. learn how these scientific discoveries changed the world..

The only constant is change. At least, that’s what the Greek philosopher Heraclitus is credited to have said. And while science and philosophy don’t always go hand in hand, there is some truth to Heraclitus’ notion. Change is inevitable and, in some cases, necessary for our species to evolve . While some change happens automatically, like the tides going in and out, some changes bloomed from scientific discoveries.
Using fire to cook food and keep warm propelled our ancestors toward the foundations of early settlements and continued the growth of civilization. Using fire to shape metals for weapons and building materials led to more and more discoveries and more and more advancements. While many advances shaped humanity, we’ve focused on ten significant scientific discoveries that changed the world.
The discovery of DNA didn’t so much change the world as it did our understanding of it — more so, our understanding of life. DNA is a term we’ve only started using in the 20th century, though its initial discovery dates back decades into the 19th century.
What Is DNA?
DNA is the molecule that encodes genetic information for all living things. It plays a key role in passing traits from parents to offspring and is the primary component of chromosomes in the cell nuclei of complex organisms.
Who Discovered DNA?
Many people think scientists James Watson and Francis Crick discovered DNA in the 1950s. Nope, not so fast. DNA was actually first discovered in 1869 by Swiss physician Friedrich Miescher . He identified what he referred to as “nuclein” in blood cells. Several other researchers have worked on projects around identifying DNA up until Watson and Crick.
What Does DNA Stand For?
The term nuclein eventually evolved into what we know as DNA, the shorthand for deoxyribonucleic acid. German biochemist Albrecht Kossel , who would later go on to win the Nobel Prize, is often credited with the name.
Levene’s Polynucleotide Model
Other scientists, such as Phoebus Levene , built on Miescher’s work over the years. Levene didn't know how DNA's nucleotide components were arranged. He proposed the polynucleotide model, correctly suggesting that nucleic acids are chains of nucleotides, each with a base, a sugar, and a phosphate group.
Watson and Crick's Double-Stranded Helix
Watson and Crick and “their” groundbreaking discovery in the field of genetics accurately identified DNA’s double-stranded helix structure, connected by hydrogen bonds. For their discovery, Watson and Crick won a Nobel Prize in 1962 and worldwide acclaim.
Though Watson and Crick won a Nobel Prize, years later, we’ve learned that the duo likely took research without permission from chemist Rosalind Franklin . Thanks to her research, the double helix structure was realized, though her Nobel Prize was not.
In 2014, Watson auctioned off his Nobel Prize medal for over $4 million. The buyer was a Russian billionaire who returned it to Watson a year later. In 2019, Watson was stripped of his honorary titles because of racist comments.
Read More: DNA in Unlikely Places Helps Piece Together Ancient Humans' Family Trees
2. Earth in Motion
While it may be common knowledge that Earth spins on an axis and revolves around the sun, at one point, this idea was extremely outlandish. How could the planet move and we not feel it? Thanks to a few clever scientists, the Earth in Motion theory became more than a wild idea.
What Is Earth in Motion?
Earth in motion refers to the understanding that Earth is not stationary but moves in different ways. Earth rotates on an axis and revolves around a star.
Earth’s Rotation
Earth rotates on its axis , which is an imaginary line running from the North Pole to the South Pole. This rotation is responsible for the day-night cycle, with one complete rotation taking about 24 hours.
Earth’s Revolution
Earth revolves around the Sun, completing one orbit approximately every 365 days. This revolution, combined with the tilt of the Earth's axis, leads to the changing seasons.
Who Discovered Earth's Motion?
The discovery and acceptance of Earth's motion was a gradual process involving several key figures in the history of science.
Aristarchus Hypothesis of Earth’s Motion
An ancient Greek astronomer, Aristarchus of Samos, was one of the first to suggest that Earth orbits the Sun . This view was not widely accepted in his time as it was believed Earth was the center of the Universe, and stars, planets, and the sun all revolved around our planet.
Copernicus Creates the First Model of Earth’s Motion
Mathematician and astronomer Nicolaus Copernicus is often credited with proposing the first heliocentric model of the universe. In 1543, he published his great work, On the Revolutions of the Heavenly Spheres , which explained his theories.
Among them was that day and night was created by the Earth spinning on its axis. Copernican heliocentrism replaced the conventionally accepted Ptolemaic theory , which asserted that the Earth was stationary. Copernicus’ work was largely unknown during his lifetime but later gained support.
Galileo Galilei’s Telescopic Observations
Galileo Galilei agreed with Copernicus’ theory and proved it through his telescopic observations. In 1610, he observed phases of Venus and the moons of Jupiter, which were strong evidence against the Earth-centered model of the universe.
Galileo agreed with Copernicus’ theory and proved it by using a telescope to confirm that the different phases Venus went through resulted from orbiting around the sun.
Johannes Kepler’s Planetary Laws
German mathematician Johannes Kepler formulated a series of laws detailing the orbits of planets around the Sun. These laws, which remain relevant today, provided mathematical equations for accurately predicting planetary movements in line with the Copernican theory.
Why Don’t We Feel Earth Spinning?
According to researchers at the California Institute of Technology (CalTech), Earth spins smoothly and at a consistent speed. If Earth were to change speeds at any time, we’d feel it.
Read More: Earth's Rotation Has Slowed Down Over Billions of Years
3. Electricity
Did benjamin franklin discover electricity.
It’s a common misconception that Ben Franklin discovered electricity with his famous kite experiment. But his 1752 experiment, which used a key and kite, instead demonstrated that lightning is a form of electricity . Another myth is that Franklin was struck by lightning. He wasn’t, but the storm did charge the kite.
Who First Observed Electricity?
Back in 600 B.C.E., it was the ancient Greek philosopher Thales of Miletus who first observed static electricity when fur was rubbed against fossilized tree resin, known as amber.
Who Invented Electricity?
British scientist and doctor William Gilbert coined the word “electric,” derived from the Greek word for amber. Regarded as the “father of electricity,” Gilbert was also the first person to use the terms magnetic pole, electric force, and electric attraction. In 1600, his six-volume book set, De Magnete , was published. Among other ideas, it included the hypothesis that Earth itself is a magnet.
Read More: Ben Franklin: Founding Father, Citizen Scientist
4. Germ Theory of Disease
What is the germ theory of disease.
Germ theory is a scientific principle in medicine that attributes the cause of many diseases to microorganisms, such as bacteria and viruses, that invade and multiply within the human body. This theory was a significant shift from previous beliefs about disease causation.
Who Invented the Germ Theory?
Louis Pasteur discovered germ theory when he demonstrated that living microorganisms caused fermentation , which could make milk and wine turn sour. From there, his experiments revealed that these microbes could be destroyed by heating them — a process we now know as pasteurization.
This advance was a game changer, saving people from getting sick from the bacteria in unpasteurized foods , such as eggs, milk, and cheeses. Before Pasteur, everyday people and scientists alike believed that disease came from inside the body.
Pasteur’s work proved that germ theory was true and that disease was the result of microorganisms attacking the body. Because of Pasteur, attitudes changed, and became more accepting of germ theory.
How Did Koch’s Postulates Contribute to Germ Theory?
The German physician and microbiologist Robert Koch played a crucial role in establishing a systematic methodology for proving the causal relationship between microbes and diseases .
He formulated Koch's postulates and applied these principles to identify the bacteria responsible for tuberculosis and cholera, among other diseases.
Together, Pasteur and Koch laid the foundation for bacteriology as a science and dramatically shifted the medical community's understanding of infectious diseases. Their work led to improved hygiene, the development of vaccines, and the advancement of public health measures.
Read More: Why Do Some People Get Sick All the Time, While Others Stay in Freakishly Good Health?
Who Discovered Gravity?
Isaac Newton didn’t really get hit on the head with an apple, as far as we know. But seeing an apple fall from a tree did spark an idea that would lead the mathematician and physicist to discover gravity at the age of just 23.
He pondered about how the force pulls objects straight to the ground, as opposed to following a curved path, like a fired cannonball. Gravity was the answer — a force that pulls objects toward each other.
How Does Gravity Work?
The greater the mass an object has, the greater the force or gravitational pull. When objects are farther apart, the weaker the force. Newton’s work and his understanding of gravity are used to explain everything from the trajectory of a baseball to the Earth’s orbit around the sun. But Newton’s discoveries didn’t stop there.
Newton’s Laws of Motion
In 1687, Newton published his book Principia , which expanded on his laws of universal gravitation and his three laws of motion. His work laid the foundation for modern physics.
Building on the discovery, advancements in the field of electricity continued.
In 1800, Italian physicist Alessandro Volta created the first voltaic pile , an early form of an electric battery.
Einstein’s Theory of General Relativity
In 1915, Einstein proposed the theory of general relativity . This theory redefined gravity not as a force but as a curvature of spacetime caused by the presence of mass and energy.
According to Einstein, massive objects cause a distortion in the fabric of space and time, similar to how a heavy ball placed on a trampoline causes it to warp. Other objects move along the curves in spacetime created by this distortion.
Both Newton and Einstein significantly advanced our understanding of gravity. Their theories marked critical milestones in the field of physics and have had far-reaching implications in science and technology.
Read More: 5 Eccentric Facts About Isaac Newton
6. Antibiotics
Much like Germ Theory revolutionized modern medicine, so too did the invention of antibiotics. This discovery would go on to save countless lives.
When Were Antibiotics Invented?
According to the Microbiology Society , humans have been using some form of antibiotics for millennia. It was only in recent history that humans realized that bacteria caused certain infections and that we could now provide readily available treatment.
In 1909, German physician Paul Ehrlich noticed that certain chemical dyes did not color certain bacteria cells as it did for others. Because of this, he believed that it would be possible to kill certain bacteria without killing the other cells around it. Ehrlich went on to discover the cure for syphilis, which many in the scientific community refer to as the first antibiotic. However, Ehrlich referred to his discovery as chemotherapy because it used chemicals to treat a disease. Ehrlich is referred to as the “Father of Immunology” for his discoveries.
Ukrainian-American microbiologist Selman Waksman coined the term “antibiotic” about 30 years later, according to the Microbiology Society.
Who Discovered Penicillin?
One of the most recognizable antibiotics known today is penicillin. Health professionals prescribe millions of patients with this antibiotic each year. However, one of the most well-known antibiotics was discovered by accident.
In 1928, after some time away from the lab, Alexander Fleming — a Scottish microbiologist — discovered that a fungus Penicillium notatum had contaminated a culture plate with Staph bacteria. Fleming noticed that the fungus had created bacteria-free areas on the plate. After multiple trials, Fleming was able to successfully prove that P. notatum prevented the growth of Staph. Soon the antibiotic was ready for mass production and helped save many lives during World War Two.
What Is Penicillin Used For?
Penicillin is used to treat infections caused by bacteria. The medication works by stopping and preventing the growth of bacteria.
Read More: Antibiotic-Resistant Bacteria: What They Are and How Scientists Are Combating Them
7. The Big Bang Theory
The Big Bang Theory is one of the most widely accepted theories on the beginning of the universe. The theory claims that about 13.7 billion years ago, all matter of the universe was condensed into one small point. After a massive explosion, the contents of the universe burst forth and expanded and continue to expand today.
Who Came Up With the Big Bang Theory?
This first mention of the Big Bang came from Georges Lemaître, a Belgian cosmologist and Catholic priest. Initially, in 1927, Lemaître published a paper about General Relativity and solutions to the equations around it. Though it mostly went unnoticed.
Though many scientists didn’t believe that the universe was expanding, a group of cosmologists was beginning to go against the grain. After Edwin Hubble noticed that galaxies further away from our own seemed to be pulling away faster than those closer to us, the idea of the universe expanding seemed to make more sense. Lemaître’s 1927 paper was recognized, and the term Big Bang appeared in Lemaître’s 1931 paper on the subject.
What Is the Hubble Space Telescope?
Edwin Hubble’s discovery that galaxies are moving away from our own, dubbed Hubble’s Law, is on a long list of his many discoveries. Though this discovery helped add evidence to the Big Bang Theory, this discovery was hindered by the same thing that had been distributing telescopes since their inception: Earth’s atmosphere. According to NASA , Earth’s atmosphere distorts light, limiting how far a telescope can see, even on a clear night.
Because of this, researchers, specifically Lyman Spitzer , suggested putting a telescope in space, just beyond Earth’s atmosphere and into its orbit. After a few attempts in the 1960s and 70s, NASA, along with contributions from the European Space Agency (ESA), launched a space telescope on April 24, 1990 . The Hubble Space Telescope, named for the pioneering cosmologist, became the strongest telescope known to humankind until the 2021 launch of the James Webb Space Telescope .
What Is The Cosmic Microwave Background?
The Big Bang emitted large amounts of primeval light , according to the ESA. Over time, this light “cooled” and was no longer visible. However, researchers are able to detect what is known as Cosmic Microwave Background (CMB), which is, according to the ESA, the cooled remnant of the first light to travel through the universe. Some researchers even refer to CMB as an echo of the Big Bang.
Read More: Did the Big Bang Happen More Than Once?
8. Vaccines
“An ounce of prevention is worth a pound of cure,” Benjamin Franklin once said. A statement that, at the time, applied to making towns safer against fires. However, the same statement can be true for health and wellness. The advent of vaccines has helped prevent several serious diseases and keep people safe. Thanks to vaccines, people rarely get diseases like polio, and smallpox has been eradicated .
What Is a Vaccine?
According to the Centers for Disease Control (CDC), a vaccine is a method of protection that introduces a small amount of disease to the human body so that the body can form an immune response should that disease try to enter the body again.
Basically, through a vaccine, the human body is exposed to a small out of a disease so that the immune system can build a defense against it.
When Was the First Vaccine Created?
According to the World Health Organization (WHO), Dr. Edward Jenner created the first vaccine in 1796 by using infected material from a cowpox sore — a disease similar to smallpox. He inoculated an 8-year-old boy named James Phipps with the matter and found that the boy, though he didn’t feel well at first, recovered from the illness.
A few months later, Jenner tested Phipps with material from a smallpox sore and found that Phipps did not get ill at all. From there, the smallpox vaccine prevented countless deaths in the centuries to come.
When Was the Polio Vaccine Invented?
From 1796 to 1945, doctors and scientists worked hard to create vaccines for other serious illnesses like the Spanish Flu, yellow fever, and influenza. One of these doctors was Jonas Salk. After Salk helped develop an influenza vaccine in 1945, he began working on the Polio vaccine. Between 1952 and 1955, Salk finished the vaccine, and clinical trials began. Salk’s vacation method required a needle and syringe, though, by 1960, Albert Sabin had created a different delivery method for the polio vaccine. Sabin’s version could be administered by drops or on a sugar cube.
Read More: The History of the Polio Vaccine
9. Evolution
What is evolution .
Evolution is a theory that suggests that organisms change and adapt to their environment on a genetic level from one generation to the next. This can take millions of years through methods such as natural selection. An animal’s color or beak may alter over time depending on the changes in their environment, helping them hide from predators or better capture prey.
Who Is the Father of Evolution?
After studying animals in the Galapagos , particularly the finches, a naturalist named Charles Darwin determined that the birds — who all resided on different Galapagos islands — were the same or similar species but had distinct characteristics. Darwin noted that the finches from each island had different beaks. These beaks helped the finches forage for their main food source on their specific island. Some had larger beaks for cracking open nuts and seeds, while others had smaller and more narrow beaks for finding insects.
These observations earned Charles Darwin the title of the Father of Evolution. Though the theory of evolution has changed since Darwin published On the Origin of Species in 1859, he helped lay the framework for modern scientists.
Is Evolution a Theory or Fact?
The long-held belief for thousands of years was that the world and all of its organisms were created by one power. But, as science has advanced, there is clear evidence to argue against that.
The answer to this question is complicated because evolution is both fact and theory. According to the National Center for Science Education , scientific understanding needs both theories and facts. There is proof that organisms have changed or evolved over time, and scientists now have the means to study and identify how those changes happen.
Read More : 7 Things You May Not Know About Charles Darwin
What Does CRISPR Stand For?
According to the National Human Genome Research Institute, CRISPR stands for Clustered Regularly Interspaced Short Palindromic Repeats. Researchers use this technology to modify the DNA of a living organism.
Who Discovered CRISPR?
There are several people involved and decades of research into the discovery of CRISPR . These researchers include Yoshizumi Ishino, Francisco Mojica, and the duo who recently won the Nobel Prize in Chemistry for CRISPR, Jennifer Doudna and Emmanuelle Charpentier.
What Is CRISPR?
CRISPR is a technology that can edit genes or even turn a gene “on” or “off.” Researchers have described CRISPR as a molecular scissors that clips apart DNA, then replaces, deletes, or modifies genes. According to a 2018 study, scientists can use this technology to help replace certain genes that may cause diseases such as cancer or heritable diseases like Duchenne muscular dystrophy — a degenerative disorder that can cause premature death.
How Does CRISPR Work?
In short, scientists use CRISPR technology to find specific pieces of DNA inside of a cell. Scientists then alter that piece of DNA or replace it with a different DNA sequence. CRISPR technology also ensures that the changed gene passes on to the next offspring through gene drive.
Read More: CRISPR Gene-Editing Technology Enters the Body — and Space
This article was originally published on Oct. 22, 2021 and has since been updated with new information from the Discover staff.
- earth science
Already a subscriber?
Register or Log In

Keep reading for as low as $1.99!
Sign up for our weekly science updates.
Save up to 40% off the cover price when you subscribe to Discover magazine.

The 11 Most Influential Psychological Experiments in History
The history of psychology is marked by groundbreaking experiments that transformed our understanding of the human mind. These 11 Most Influential Psychological Experiments in History stand out as pivotal, offering profound insights into behaviour, cognition, and the complexities of human nature.
In this PsychologyOrg article, we’ll explain these key experiments, exploring their impact on our understanding of human behaviour and the intricate workings of the mind.
Table of Contents
Experimental psychology.
Experimental psychology is a branch of psychology that uses scientific methods to study human behaviour and mental processes. Researchers in this field design experiments to test hypotheses about topics such as perception, learning, memory, emotion, and motivation.
They use a variety of techniques to measure and analyze behaviour and mental processes, including behavioural observations, self-report measures, physiological recordings, and computer simulations. The findings of experimental psychology studies can have important implications for a wide range of fields, including education, healthcare, and public policy.
Experimental Psychology, Psychologists have long tried to gain insight into how we perceive the world, to understand what motivates our behavior. They have made great strides in lifting that veil of mystery. In addition to providing us with food for stimulating party conversations, some of the most famous psychological experiments of the last century reveal surprising and universal truths about nature.

Throughout the history of psychology, revolutionary experiments have reshaped our comprehension of the human mind. These 11 experiments are pivotal, providing deep insights into human behaviour, cognition, and the intricate facets of human nature.
1. Kohler and the Chimpanzee experiment
Wolfgang Kohler studied the insight process by observing the behaviour of chimpanzees in a problem situation. In the experimental situation, the animals were placed in a cage outside of which food, for example, a banana, was stored. There were other objects in the cage, such as sticks or boxes. The animals participating in the experiment were hungry, so they needed to get to the food. At first, the chimpanzee used sticks mainly for playful activities; but suddenly, in the mind of the hungry chimpanzee, a relationship between sticks and food developed.
The cane, from an object to play with, became an instrument through which it was possible to reach the banana placed outside the cage. There has been a restructuring of the perceptual field: Kohler stressed that the appearance of the new behaviour was not the result of random attempts according to a process of trial and error. It is one of the first experiments on the intelligence of chimpanzees.
2. Harlow’s experiment on attachment with monkeys
In a scientific paper (1959), Harry F. Harlow described how he had separated baby rhesus monkeys from their mothers at birth, and raised them with the help of “puppet mothers”: in a series of experiments he compared the behavior of monkeys in two situations:
Little monkeys with a puppet mother without a bottle, but covered in a soft, fluffy, and furry fabric. Little monkeys with a “puppet” mother that supplied food, but was covered in wire. The little monkeys showed a clear preference for the “furry” mother, spending an average of fifteen hours a day attached to her, even though they were exclusively fed by the “suckling” puppet mother. conclusions of the Harlow experiment: all the experiments showed that the pleasure of contact elicited attachment behaviours, but the food did not.
3. The Strange Situation by Mary Ainsworth
Building on Bowlby’s attachment theory, Mary Ainsworth and colleagues (1978) have developed an experimental method called the Strange Situation, to assess individual differences in attachment security. The Strange Situation includes a series of short laboratory episodes in a comfortable environment and the child’s behaviors are observed.
Ainsworth and colleagues have paid special attention to the child’s behaviour at the time of reunion with the caregiver after a brief separation, thus identifying three different attachment patterns or styles, so called from that moment on. kinds of attachment according to Mary Ainsworth:
Secure attachment (63% of the dyads examined) Anxious-resistant or ambivalent (16%) Avoidant (21%) The Strange Situation by Mary Ainsworth
In a famous 1971 experiment, known as the Stanford Prison, Zimbardo and a team of collaborators reproduced a prison in the garages of Stanford University to study the behaviour of subjects in a context of very particular and complex dynamics. Let’s see how it went and the thoughts on the Stanford prison experiment. The participants (24 students) were randomly divided into two groups:
“ Prisoners “. The latter were locked up in three cells in the basement of a University building for six days; they were required to wear a white robe with a paper over it and a chain on the right ankle. “ Guards “. The students who had the role of prison guards had to watch the basement, choose the most appropriate methods to maintain order, and make the “prisoners” perform various tasks; they were asked to wear dark glasses and uniforms, and never to be violent towards the participants of the opposite role. However, the situation deteriorated dramatically: the fake police officers very soon began to seriously mistreat and humiliate the “detainees”, so it was decided to discontinue the experiment.
4. Jane Elliot’s Blue Eyes Experiment
On April 5, 1968, in a small school in Riceville, Iowa, Professor Jane Elliot decided to give a practical lesson on racism to 28 children of about eight years of age through the blue eyes brown eyes experiment.
“Children with brown eyes are the best,” the instructor began. “They are more beautiful and intelligent.” She wrote the word “melanin” on the board and explained that it was a substance that made people intelligent. Dark-eyed children have more, so they are more intelligent, while blue-eyed children “go hand in hand.”
In a very short time, the brown-eyed children began to treat their blue-eyed classmates with superiority, who in turn lost their self-confidence. A very good girl started making mistakes during arithmetic class, and at recess, she was approached by three little friends with brown eyes “You have to apologize because you get in their way and because we are the best,” said one of them. The girl hastened to apologize. This is one of the psychosocial experiments demonstrating how beliefs and prejudices play a role.
5. The Bobo de Bbandura doll
Albert Bandura gained great fame for the Bobo doll experiment on child imitation aggression, where:
A group of children took as an example, by visual capacity, the adults in a room, without their behaviour being commented on, hit the Bobo doll. Other contemporaries, on the other hand, saw adults sitting, always in absolute silence, next to Bobo.
Finally, all these children were brought to a room full of toys, including a doll like Bobo. Of the 10 children who hit the doll, 8 were those who had seen it done before by an adult. This explains how if a model that we follow performs a certain action, we are tempted to imitate it and this happens especially in children who still do not have the experience to understand for themselves if that behaviour is correct or not.
6. Milgram’s experiment
The Milgram experiment was first carried out in 1961 by psychologist Stanley Milgram, as an investigation into the degree of our deference to authority. A subject is invited to give an electric shock to an individual playing the role of the student, positioned behind a screen when he does not answer a question correctly. An authorized person then tells the subject to gradually increase the intensity of the shock until the student screams in pain and begs to stop.
No justification is given, except for the fact that the authorized person tells the subject to obey. In reality, it was staged: there was absolutely no electric shock given, but in the experiment two-thirds of the subjects were influenced by what they thought was a 450-volt shock, simply because a person in authority told them they would not be responsible for it. nothing.
7. little Albert
We see little Albert’s experiment on unconditioned stimulus, which must be the most famous psychological study. John Watson and Rosalie Raynor showed a white laboratory rat to a nine-month-old boy, little Albert. At first, the boy showed no fear, but then Watson jumped up from behind and made him flinch with a sudden noise by hitting a metal bar with a hammer. Of course, the noise frightened little Albert, who began to cry.
Every time the rat was brought out, Watson and Raynor would rattle the bar with their hammer to scare the poor boy away. Soon the mere sight of the rat was enough to reduce little Albert to a trembling bundle of nerves: he had learned to fear the sight of a rat, and soon afterwards began to fear a series of similar objects shown to him.
8. Pavlov’s dog
Ivan Pavlov’s sheepdog became famous for his experiments that led him to discover what we call “classical conditioning” or “Pavlovian reflex” and is still a very famous psychological experiment today. Hardly any other psychological experiment is cited so often and with such gusto as Pavlov’s theory expounded in 1905: the Russian physiologist had been impressed by the fact that his dogs did not begin to drool at the sight of food, but rather when they heard it. to the laboratory employees who took it away.
He researched it and ordered a buzzer to ring every time it was mealtime. Very soon the sound of the doorbell was enough for the dogs to start drooling: they had connected the signal to the arrival of food.
9. Asch’s experiment
It is about a social psychology experiment carried out in 1951 by the Polish psychologist Solomon Asch on the influence of the majority and social conformity.
The experiment is based on the idea that being part of a group is a sufficient condition to change a person’s actions, judgments, and visual perceptions. The very simple experiment consisted of asking the subjects involved to associate line 1 drawn on a white sheet with the corresponding one, choosing between three different lines A, B, and C present on another sheet. Only one was identical to the other, while the other two were longer or shorter.
The experimentation was carried out in three phases. As soon as one of the subjects, Asch’s accomplice gave a wrong answer associating line 1 with the wrong one, the other members of the group also made the same mistake, even though the correct answer was more than obvious. The participants questioned the reason for this choice and responded that aware of the correct answer, they had decided to conform to the group, adapting to those who had preceded them.
psychotherapy definition types and techniques | Psychotherapy vs therapy Psychologyorg.com
10. Rosenbaum’s experiment
Among the most interesting investigations in this field, an experiment carried out by David Rosenhan (1923) to document the low validity of psychiatric diagnoses stands out. Rosenhan admitted eight assistants to various psychiatric hospitals claiming psychotic symptoms, but once they entered the hospital they behaved as usual.
Despite this, they were held on average for 19 days, with all but one being diagnosed as “psychotic”. One of the reasons why the staff is not aware of the “normality” of the subjects, is, according to Rosenhan, the very little contact between the staff and the patients.
11. Bystander Effect (1968)
The Bystander Effect studied in 1968 after the tragic case of Kitty Genovese, explores how individuals are less likely to intervene in emergencies when others are present. The original research by John Darley and Bibb Latané involved staged scenarios where participants believed they were part of a discussion via intercom.
In the experiment, participants were led to believe they were communicating with others about personal problems. Unknown to them, the discussions were staged, and at a certain point, a participant (confederate) pretended to have a seizure or needed help.
The results were startling. When participants believed they were the sole witness to the emergency, they responded quickly and sought help. However, when they thought others were also present (but were confederates instructed to not intervene), the likelihood of any individual offering help significantly decreased. This phenomenon became known as the Bystander Effect.
The diffusion of responsibility, where individuals assume others will take action, contributes to this effect. The presence of others creates a diffusion of responsibility among bystanders, leading to a decreased likelihood of any single individual taking action.
This experiment highlighted the social and psychological factors influencing intervention during emergencies and emphasized the importance of understanding bystander behaviour in critical situations.
The journey through the “11 Most Influential Psychological Experiments in History” illuminates the profound impact these studies have had on our understanding of human behaviour, cognition, and social dynamics.
Each experiment stands as a testament to the dedication of pioneering psychologists who dared to delve into the complexities of the human mind. From Milgram’s obedience studies to Zimbardo’s Stanford Prison Experiment, these trials have shaped not only the field of psychology but also our societal perceptions and ethical considerations in research.
They serve as timeless benchmarks, reminding us of the ethical responsibilities and the far-reaching implications of delving into the human psyche. The enduring legacy of these experiments lies not only in their scientific contributions but also in the ethical reflections they provoke, urging us to navigate the boundaries of knowledge with caution, empathy, and an unwavering commitment to understanding the intricacies of our humanity.
What is the most famous experiment in the history of psychology?
One of the most famous experiments is the Milgram Experiment, conducted by Stanley Milgram in the 1960s. It investigated obedience to authority figures and remains influential in understanding human behaviour.
Who wrote the 25 most influential psychological experiments in history?
The book “The 25 Most Influential Psychological Experiments in History” was written by Michael Shermer, a science writer and historian of science.
What is the history of experimental psychology?
Experimental psychology traces back to Wilhelm Wundt, often considered the father of experimental psychology. He established the first psychology laboratory in 1879 at the University of Leipzig, marking the formal beginning of experimental psychology as a distinct field.
What was the psychological experiment in the 1960s?
Many significant psychological experiments were conducted in the 1960s. One notable example is the Stanford Prison Experiment led by Philip Zimbardo, which examined the effects of situational roles on behaviour.
Who was the first experimental psychologist?
Wilhelm Wundt is often regarded as the first experimental psychologist due to his establishment of the first psychology laboratory and his emphasis on empirical research methods in psychology.
If you want to read more articles similar to The 11 Most Influential Psychological Experiments in History , we recommend that you enter our Psychology category.
I'm Waqar, a passionate psychologist and dedicated content writer. With a deep interest in understanding human behavior, I aim to share insights and knowledge in the field of psychology through this blog. Feel free to reach out for collaborations, queries, or discussions. Let's dig into the fascinating world of psychology together!
Similar Posts

What are Positive and Negative Emotions
During the course of the day, there are many emotions that we can experience. Emotions are part of the natural condition of the person and these…

Psychology of Anime: How It Shapes Our Minds
Anime, a genre of hand-drawn, animated films originating from Japan, has gained widespread global popularity, entrancing audiences with its unique art style and characters often…

What is Weber’s law in psychology? Explained
Weber’s Law, formulated by the German physiologist Ernst Heinrich Weber in the 19th century, is a fundamental principle in psychology and psychophysics. It is an…

journal of personality and social psychology
The Journal of Personality and Social Psychology (JPSP) is a renowned scientific publication that delves into the intricate workings of human behavior. With its extensive…

Psychology of not calling someone by their name
In our intricate web of human interactions, a seemingly inconspicuous behavior often holds more significance than meets the eye: The psychology of not calling someone…

The psychology behind cheating and lying
1. Introduction Cheating and lying are complex behaviors that can have significant psychological implications. They often stem from various underlying motivations and can have profound…
Leave a Reply Cancel reply
You must be logged in to post a comment.
- Testimonials
- Publications
- Class Schedule
- Terms and Conditions
- JC Syllabus
- IP/Secondary
The Top 10 Greatest Physics Experiments Of All Time

Experiments form the backbone of science. It is through experiments that many theories in science have been discovered, proved and evolved into fundamental facts. While it is true that most of the science we have today is theoretical, it was experiments along histories that led to major milestones in science, especially in Physics.
Physics tuition in Singapore will enhance student’s interest when they learn about experiments that led to major milestones in Physics. Here are the top 10 greatest physics experiments of all time that have shed light on some of the mysteries that surround us.
1. Galileo Galilei’s Experiment on Speed of Falling Objects
Before Galileo, Aristotle had argued that heavy objects fall at a faster rate than lighter objects. But Galileo who is famed for his work on gravity, motion and light proved that objects fall at the same speed irrespective of their weight. He demonstrated this with a stone and feather experiment.
2. James Prescott Joule‘s Energy Conservation Experiment
This experiment conducted in 1840 demonstrated that conservation of energy is nothing but a conversion of energy from one form to another. He found that Energy lost = energy gained is another way and you can’t create or destroy energy, but you can change it from one form into another.
3. Isaac Newton’s Discovery of Light Spectrum
Isaac Newton placed a triangle glass (prism) along his window and split sunlight into many colors, this experiment done in 1672 is a landmark revelation that led onto many other interesting and essential discoveries about the light spectrum and its properties.

4. Henry Cavendish’s Earth Density Experiment
Demonstrated in 1798, this popular experiment with only 4 balls and 2 sticks achieved the impossible task of weighing the world via the measurement of Earth’s density.
5. Hippolyte Fizeau’s Experiment on Light Speed
In 1851, Fizeau managed to measure the speed at which light travels using a simple gear wheel and mirrors. Thou it is approximately 5% off the actual speed, it’s amazing at that time and has built a foundation for Léon Foucault whom improved the experiment and more accurately calculated the speed of light. Most of the mysteries of physics are easily cracked with simple experiments just as Fizeau’s.
6.Robert Millikan’s Measurement of the Charge of Electron
In 1909, American scientist Millikan found that the measure of an electron’s charge could be evaluated by using metal plates, transparent chamber, and a few oil drops. By measuring the velocity between power off and on, he discovered the charge on the electron and won himself the Nobel Prize in Physics.
7. Enrico Fermi’s Nuclear Chain Reaction Experiment
In 1942, Enrico Fermi fired an uncharged neutron to Uranium 235 to form Uranium 236, the additional neutrons generated went on to hit other Uranium 235, causing a nuclear chain reaction, which has unearthed potentially dangerous and at the same time beneficial revelations about nuclear energy . E=MC^2 is the famous formula responsible for this chain reaction.
8. Rutherford’s Gold Foil Experiment
In 1909, Rutherford with the aid of Geiger and Marsden, discovered the atomic structure as we know it today with a core nucleus and electrons around it by firing positively charged particles at a sheet of gold foil.
9. Rosalind Franklin DNA Radiographs
Franklin’s photo of X-ray diffraction had thrown light on immense and critical information regarding DNA structure using a similar concept to shadow puppetry.
10. Foucault’s Pendulum Experiment
This experiment done in 1851 in Paris demonstrated the all-important revolving movement of earth on its own axis. Researchers have since confirmed about 24 hours as the oscillation period. As you can see that what we have taken for granted about various elements around us has actually been discovered through several trial and errors by great physicists. Being tutored in JC Physics tuition will give students a great opportunity to learn in-depth all they need to know about physics and more, so they shine in the subject and score high grades.
TuitionPhysics
LinkedIn Profile
Copyright: Best Physics Tuition™ Centre. All Rights Reserved | User Sitemap
- The Impact of Sleep Deprivation on Health and Well-being
- Premarital Counseling: Insights from Psychologists for a Stronger Marriage
- The First Step: 6 Things to Know Going Into Your First Therapy Appointment
- Big Four Auditing Firms have incorporated New Employee Well-Being Initiatives
- Neuroscience of Motivation: How Orexin Neurons Drive Voluntary Exercise and Resist Temptation

Your cart is empty

10 Great Experiments in the field of Psychology
- by Psychologs Magazine
- November 1, 2023
- 6 minutes read

A scientific research that tests a theory is called an experiment. In an experiment, one manipulates an independent variable (the cause), measures the dependent variable (the effect), and controls any unimportant variables. The fact that experiments should be unbiased is a benefit. A study that closely follows a scientific research plan is called an experimental research study. It consists of a hypothesis, a variable that the researcher can change, and variables that are calculable, measurable, and comparable. The fact that experimental research is conducted in a controlled setting is crucial.
Read More: 15 Women psychologists Who made their contribution to the field
We can make better decisions regarding our ideas and projects when we experiment. People frequently make the error of taking their concept and running with it without verifying the underlying presumptions. We often imagine we know something when in fact we don’t know. Let’s talk about the AIM we write in an experiment- An experiment’s objective is its goal. It states what can be inferred from the experiment, to put it another way. “To see how light is affected by lenses and plates of glass of various thicknesses.” The goal needs to be one or two lines in length.
Types of Experiments
A. field experiment.
A field experiment is a psychology research method that is conducted in an organic, real-world environment. In that the researcher modifies one or more independent variables and assesses the impact on the dependent variable, it is comparable to a laboratory experiment. In contrast, the subjects in a field experiment are not aware that they are being observed, and the researcher has less control over the unrelated variables.
Field experiments are a popular tool for researching social issues like persuasion, obedience, and altruism. They are also employed in evaluating the efficacy of interventions in practical contexts, like public health campaigns and educational initiatives.
B. Lab Experiment
In psychology, a laboratory experiment is a type of study where researchers modify one or more independent variables and then observe how those changes affect the dependent variable under carefully monitored circumstances. A laboratory experiment is carried out in extremely controlled settings where precise measurements can be made – though a laboratory is not always necessary. To decide on the location, timing, participants, and conditions of the experiment, the researcher employs a standard operating procedure. Random assignments are made to each group based on an independent variable.
C. Natural Experiment
In psychology, a natural experiment is a type of study where the researcher examines, without changing any variables, how a naturally occurring event or setting affects the dependent variable. Natural experiments take place in the participants’ daily (or real-life) environments; nevertheless, in these cases, the experimenter has no control over the independent variable because it happens in real life. Natural experiments are frequently employed to investigate psychological phenomena, such as the aftermath of natural disasters, policy changes, or social movements, that would be challenging or immoral to investigate in a laboratory setting.
10 Great Experiments That Contribute to Modern Psychology
1. milgram’s experiment:.
In the Milgram experiment, a subject’s willingness to administer a certain amount of shock served as a proxy for obedience. Even though several of the subjects became quite anxious, upset, and hostile toward the researcher, they nevertheless obeyed instructions all the way through. As Milgram’s experiment is based on Obedience, let’s understand it with real-life examples- A youngster who complies with their parent’s wishes is an example of obedience in the real world. Another illustration would be a soldier adhering to a higher-ranking officer’s orders.
2. Standford Prison Experiment:
Standford Prison experiment was based on examining the effect of role-plays, labelling and social expectations on behaviour over two weeks. In this experiment, 24 subjects were selected and were paid $ 15 a day which was divided into prisoners and guards. Few were asked to play the role of prisoners and few were asked to play of guards. Guards were given mirrored spectacles that prohibited eye contact and were instructed not to physically mistreat detainees.
Read: Some Lesser Known Fields of Psychology
The experimenters received the prisoners after they had been “arrested” by real police and placed in a makeshift jail located in a campus building’s basement. Only the second day passed before the inmates rose in rebellion. Then, to control the inmates, the guards devised a system of punishments and penalties. Three inmates were discharged after the first four days because they were so traumatized. Several of the inmates experienced depression and disorientation during the experiment, while some of the guards became vicious and oppressive.
3. Bobo Doll Experiment:
Albert Bandura, a psychologist, conducted the ground-breaking Bobo doll experiment, a research on violence that showed youngsters could pick up skills by seeing adults behave. Children who saw an adult hitting the doll during their playtime with Bobo were prone to act aggressively as well. The kids kicked, pounded, and flung the doll in the air, much like their adult role models did. There were 3 stages for conducting this experiment; A test for delayed imitation; Aggression arousal; and Modelling.
Read: The Basics of Child Psychology
4. Little Albert:
This experiment showed that a small child may be trained to dread a neutral stimulus—a stimulus that the youngster had not previously shown any fear of. This result was also extrapolated to encompass additional animals or objects with comparable appearances.
5. Asch Experiment:
The results of the trials showed how much one’s own opinions are impacted by those of a group. Asch discovered that individuals were prepared to overlook the truth and provide a false response to fit in with the group. Shown that the influence of peer pressure on our conduct is more than previously thought. It was surprising that peer pressure could persuade someone to offer an answer that was wrong based on what they could see with their own eyes.
6. Halo Effect:
A sort of cognitive bias known as the “halo effect” occurs when our general perception of someone affects our feelings and thoughts about them as a person. The “physical attractiveness stereotype” and the “what is beautiful is also good” idea are other terms used to describe the halo effect. Example- Even though a student exhibits excellent academic performance and intelligence, an unruly attitude can lead an instructor to conclude that the student is not a good student due to his lack of behaviour.
Read: The Difference Between Psychology and Psychiatry
7. Classical Conditioning:
This experiment was demonstrated by Ivan V Pavlov. In this experiment, dogs were conditioned in a way that they would salivate with the arrival of food which was associated with the ringing of the bell.
8. Cognitive Dissonance Experiment:
The experiment of Leon Festinger demonstrated that people hold negative beliefs which lead to discomfort. He explained the concept of motivation to them which will help them to reduce their discomfort.
9. The Hawthrone Studies:
This experiment talks about the importance of social and psychological factors in the work environment as various workplace conditions impact the productivity of the employees.
10. Harlow’s Monkey Experiment:
This experiment tells us about the importance of social and emotional development. It was performed on Infant monkeys who preferred to have contact with a soft, comforting surrogate mother over a wire mother that provided food. This experiment shows how individuals or animals attract more where they have emotional or social support.
A scientific method for examining human thought and behaviour in experimental psychology. To test ideas and hypotheses about different mental processes and advance our knowledge of human behaviour, experimental psychologists employ rigorous research methods such as experiments. It also tries to modify factors that could result in behaviour to explain the actions of animals (including humans) and the functional arrangement of mental processes.
Experiments help in shaping the field of Psychology which provides insights into various cognition, social dynamics and human behavior. There were many changes in research practices and regulations, which tells us that these experiments have faced ethical concerns and limitations over the years.
Share This Post:
Indian rituals and their relation to psychology, 10 common myths around psychology you didn’t know, leave feedback about this cancel reply.
- Rating 5 4 3 2 1
Related Post

Understanding The Stigma That Surrounds Mental Health

Porn Addiction and Desensitisation to Victims

Understanding Mindfulness-Based Stress Reduction (MBSR)

Sexting and Mental Health of Young Adults

Experiencing Self disgust: when the self feeds off

20 Best Science Experiments At Home
Whether you are homeschooling or just stuck at home looking for something educational and fun to do with the kids, science experiments are a fantastic choice! Not only do kids love doing these science experiments, but they are learning valuable scientific methods, vocabulary and processes that can help them throughout their lives.
Science Experiments To Do At Home
What you will discover in this article!

Disclaimer: This article may contain commission or affiliate links. As an Amazon Influencer I earn from qualifying purchases. Not seeing our videos? Turn off any adblockers to ensure our video feed can be seen. Or visit our YouTube channel to see if the video has been uploaded there. We are slowly uploading our archives. Thanks!
Lately this has become a very common theme.
Kids are bored. They really want something to do. Something interesting, exciting and fun!
Parents are trying to find ways to entertain and educate their children.
Homeschooling has become incredibly popular, but parents are struggling to find cool science experiments to do at home. Something that is suitable without breaking the bank when it comes to supplies, won’t destroy their home, and that will actually teach their kids about chemistry, biology and physics.
So with that in mind, here are the top 20 Science Experiments to do at home as chosen by not only me, but the readers here at STEAM Powered Family!
The Best At Home Science Experiments
In selecting which experiments ranked as the absolute BEST science experiments to do with your kids at home we had a few different criteria.
- They needed to be popular! These experiments are tried and true, and have loyal following of readers that LOVE doing them. Once you try them, you will know why STEAM Powered Family readers voted these as their favourite science experiments!
- Supplies must be relatively easy to source. In most cases you will likely have all the materials you need at home right now. Where you need to order or purchase a few supplies, they are readily accessible and links to purchase are included.
- They must be easy to do, with logical, easy follow directions. We are all overwhelmed. No one wants an experiment that is complex and confusing.
- The experiments need to be adaptable to wide variety of ages, grades, abilities and interests.
- The lessons need to be interesting! In all of our science experiments and STEM activities, we include a scientific explanation that either your children can read themselves, or you can read and use to help explain the experiment.
- They need to be FUN!
With that in mind, here are our top 20 picks for the BEST science experiments to do at home with your kids!
Baking Soda and Vinegar Experiments
There are so many cool experiments you can do with these pantry staples. Yes, you can do a standard volcano, but you can also hatch dino eggs, create fireworks, set off explosive bottle rockets and more! You can keep your kids busy experimenting for a ages with all of our baking soda and vinegar ideas. Get your hands on some bulk vinegar and baking soda and get experimenting!

Oobleck is like slime, but much more fascinating and sciencey! Oobleck is a non-Newtonian Fluid that becomes solid under pressure, and liquifies when pressure is removed. It is fascinating, and we have a number of different recipes, so you can find the one that uses whatever you have available in the house.
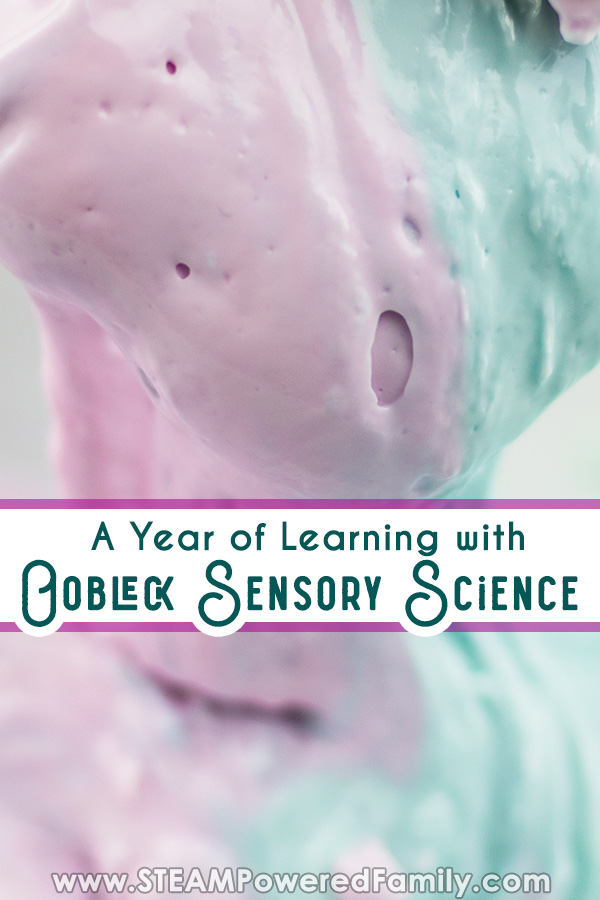
Make a Compass
This science experiment is really cool and a wonderful tie into geography and social studies lessons. Kids learn how to make a compass with materials from around the house.

Make a Lava Lamp
With 5 different ways to make a Lava Lamp, it’s no wonder our readers rate this as one of their favourite activities! Kids are fascinated by the chemical reaction that causes the mesmerizing bubbles to bob up and down in a science driven lava lamp experiment.

Grow Crystals
This is one of the most beautiful experiments we have done and always gets rave reviews from readers. Growing crystals is a wonderful science experiment that has fantastic ties to studies in geology. You can also grow edible crystals. Plus the results… STUNNING!

Ready for a fun, giggle-fest science experiment? In this science experiment we are removing the shell of a raw egg! The result is a bouncy, colourful egg. This science experiment is the perfect compliment to a study of biology and reproduction as you can also use this experiment to teach the parts of the cell and egg.
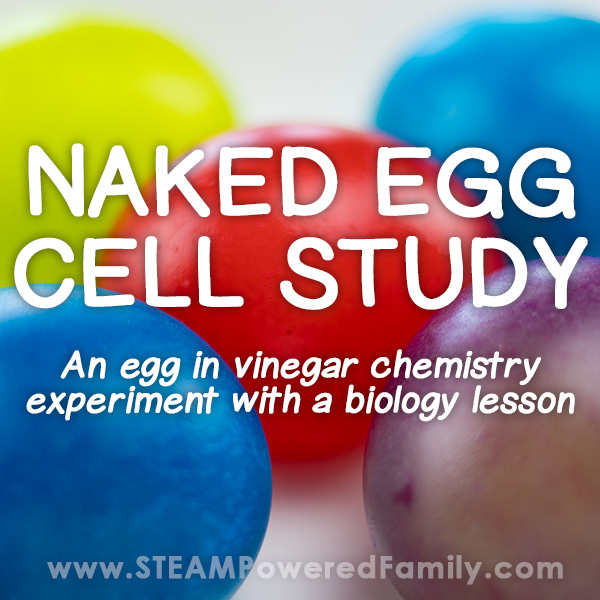
Lemon Battery
You could make a battery with lemons, squash, pumpkins, potatoes, there are lots of options! But the lemons are by far the most popular battery project. You will need some supplies, but once you have those supplies you can reuse them in lots of circuit building activities. Plus, once you are finished making your lemon battery, you can make lemon volcanoes!
Wingardium Leviosar
Whether you are a fan of Harry Potter or not, this experiment with magnets is a hit with kids. It’s like magic as they make a feather levitate!

Walking Rainbow
This classic experiment is a beautiful demonstration of primary and secondary colours, while also learning about the fascinating science behind capillary action. All you need to start your rainbow walking is cups/jars, water, food colouring and paper towels!

Make Bioplastics
Making bioplastics is an experiment that is a HUGE hit with older kids as part of their studies in polymers and environmental sciences. We have made bioplastics with both milk and gelatin, so you can pick which one to tackle based on the supplies you have on hand. Wonderful for helping kids understand how we can make plastics, and the challenges of making plastics, without fossil fuels.

Build a Heart Model
This engineering project is a fantastic way to do some studies into biology and how the heart functions. Using recycled bottles, straws, a bit of playdough and water, you can have your model pumping in no time!

Skittles Experiment
A simple, yet timeless experiment. Kids of all ages love making these gorgeous images using nothing but candy, water and the magic of science. We also used this as a chance to explore van Gogh and fluid dynamics.

So easy, yet so amazing! Magic Milk is another classic experiment that is as simple or as complex as you want to make it. I did this with my middle school kids and we had a blast exploring how the fat content of our milk affected the explosion of colours!

Slurpee Science
Heat transfer is a fascinating science to explore with your kids. If you want to make this a more serious scientific study, you can focus on the effects of salt on ice, but we like to up the fun, so we turned it into a slurpee making science project. Kids love that delicious treat made with science!

Elephant Toothpaste
Elephant Toothpaste classic experiment creating a fantastic foamy fountain that can be done safely at home using easy to source supplies and has massive wow factor for students.

Make Water Rise
Another science experiment that is a little bit like magic! Kids will learn how to create a vacuum and make water magically rise into a container. So cool!

Balloon Races
Kids got ants in their pants? Need something that is educational and will help them burn energy? Balloon races are the answer! Kids will explore physics while having a blast running about and cheering on their balloon races!

Build a Water Clock or a Windmill
Both of these projects involve some engineering and of course science but they also have some fantastic book tie ins. I LOVE projects inspired by great books!

Build a Salt Circuit
Salt circuits are a great way to introduce kids to experiments with electricity and circuitry. The supplies are minimal, and it teaches kids excellent critical thinking and problem solving skills. Plus you can make it extra fun with a glow circuit option!

Build a Catapult
A favourite that captures the excitement of every generation, and was a MUST do in our top 20 science experiments is… building catapults! We work in a little extra physics and math by turning ours into games where we need to hit targets. A definite must do for all kids.

These are 20 of our BEST picks for science experiments to do at home with your kids. This list though is far from exhaustive. Once you find something that interests your kids, search our site and see what other fun experiments you uncover. We have hundreds of experiment ideas all just waiting to inspire your kids!
Join our mailing list and we will send you even more ideas to inspire your learning!

5 Days of Smart STEM Ideas for Kids
Get started in STEM with easy, engaging activities.
- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar
- MEMBER LOGIN
Hands On As We Grow®
Hands on kids activities for hands on moms. Focusing on kids activities perfect for toddlers and preschoolers.
50 Amazingly Simple Science Experiments for Kids at Home
Science Kindergartners Preschoolers Experiment Resources 30 Comments
Kids love experimenting , and these 50 simple science experiments for kids at home from Brigitte are perfect for all ages! Plus, you probably already have the basic supplies at home.
My daughters and I have had a lot of fun doing science experiments. Each year when we create our spring and summer list , we make sure to include “science days” which are days filled with science experiments.
Sometimes our science experiments don’t work according to plan, but I have been told that all scientists have failures with experiments from time to time.
It’s okay if they aren’t all successes.
Get the FREE Science Experiments Download
50 Simple Science Experiments with Supplies You Already Have

I love these 50 simple science experiments for you to try with your little scientists. They all use basic household supplies that you probably already have at home!
Most of these are experiments my daughters and I have done together. I hope you enjoy them as much as we have!
Get little ones involved with these easy toddler-friendly science experiment ideas!

Simple Science Experiments with Water
Not only can water be a blast to play in, but water plus a few basic supplies equals a lot of science fun!
- Make an orange sink and float with an orange buoyancy experiment from Playdough to Plato.
- Compare the amount of salt in different types of water with this salty egg experiment as seen on Uplifting Mayhem.
- Do a little more sinking or floating with a fun sink or float experiment even toddlers can do from Hands On As We Grow.
- Use the free printable to record what sinks or floats in an outdoor experiment from Buggy and Buddy.
- Create some beautiful pieces of paper with this rainbow paper experiment from Science Kiddo.
- Talk about solutions as you try the “what dissolves in water” experiment as seen on Hands On As We Grow.
- Learn about water absorption with this simple experiment from Little Bins for Little Hands.
- Mix some fun colors with this oil and water experiment from Fun Learning for Kids.
- Make your own lava lamp , just like on Hands On As We Grow.
- Can you keep all the water in the bag? Try it with a leak-proof bag experiment as seen on Hands On As We Grow.
- Learn about surface tension with this magic finger pepper experiment found on Hands On As We Grow.
- Make your own water cycle in a bottle as seen on A Dab of Glue Will Do.

Simple Science Experiments with Baking Soda and Vinegar
Baking soda + vinegar = a great chemical reaction! This fizzy reaction can fuel a variety of simple science experiments at home.
First of all, we have tested and found out the absolute best combination of baking soda and vinegar to get the best reaction possible. It makes a difference if you add vinegar to baking soda or vice versa! And how much you use!
- Inflate a balloon without blowing into it with a baking soda and vinegar balloon experiment as seen on Little Bins for Little Hands.
- Practice colors as you do a baking soda and vinegar with color experiment as seen on Hands On As We Grow.
- Have fun outside with an outdoor volcano eruption as seen on Preschool Inspirations.
- Have more volcano fun by making apple volcanoes as seen on The Resourceful Mama.
- Learn about acids and bases and the chemical reaction that occurs when you make apple seeds dance with a jumping apple seeds experiment as seen on JDaniel4s Mom.
- Watch some rice dance with a dancing rice experiment as seen on Green Kid Crafts.
- Continue your dance party by making raisins dance with a dancing raisin experiment as seen on 123 Homeschool 4 Me. What other items can you get to dance?
- Learn more about acids and bases by dissolving a sea shell as seen on Teach Beside Me.
- Make an egg shell disappear with this disappearing egg activity as seen on Premeditated Leftovers.
- See how far you can launch a soda bottle with this baking soda powered boat as seen on Science Sparks.
- Make your own rocks (or eggs) with this fizzy treasure rocks experiment as seen on Living Life and Learning.
- Have some fun this summer with this frozen vinegar experiment as seen on Inspiration Laboratories.
Plant Themed Simple Science Experiments
Enjoy learning about seeds, plant parts, and how plants grow with these simple science experiments.
- Learn about how plants soak up water through their stems with a flower experiment for kids from Growing A Jeweled Rose.
- Watch seeds sprout as you grow seeds in a jar as seen on Teaching Mama.
- Learn about the parts of the seed with a seed coat experiment as seen on Gift of Curiosity.
- Build a house out of sponges and then watch it sprout with this sprout house as seen on The Stem Laboratory.
- Learn what liquids allow seeds to grow the best with this seed experiment as seen on Gift of Curiosity.
- Explore how plants grow towards the light with this shoe-box maze experiment from Plants for Kids.

Animal Themed Simple Science Experiments
Learning about animals can be even more fun with some simple hands-on simple science experiments.
- Find out more about giraffes and create some giraffe spots as seen on Preschool Powol Packets.
- Learn about how animals in the Arctic keep warm by making an arctic glove as seen on Steve Spangler Science.
- Discover how penguins stay dry with a penguin feather experiment as seen on Raising Little Superheroes.
- Learn about different bird beaks with a bird beak experiment as seen on Blessed Beyond a Doubt.
- Explore how fish (and hermit crabs) breathe with this gill experiment as seen on Preschool Powol Packets.
- Learn about sharks with a shark buoyancy experiment as seen on Little Bins for Little Hands.
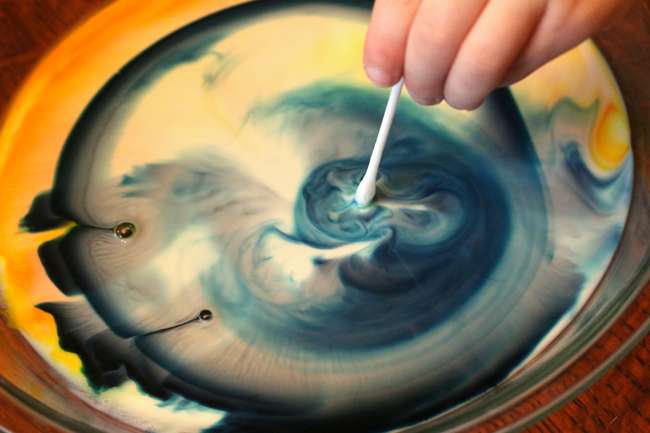
Even More Simple Science Experiment for Kids at Home!
If you are still looking for more science fun, you may enjoy the following simple science experiments.
- Find out how sugary drinks hurt teeth with an eggs-periment as seen on Feels Like Home Blog.
- Discover geodes (the state rock of Iowa) with this eggshell geode crystal experiment as seen on Science Bob.
- Learn about air pressure with an egg and bottle experiment as seen on Science Sparks.
- Find out what causes an apple to brown with this apple science experiment as seen on Teach Beside Me.
- Make an edible bubble apple with an experiment as seen on Preschool Powol Packet.
- Learn more about surface tension with a penny and water experiment as seen on Artful Parent.
- Mix colors like magic with this color changing milk experiment from Hands On As We Grow.
- Blow up a balloon with this soda and balloon experiment from Learn Play Imagine.
- Practice letters by making beautiful crystal letters as seen on Books and Giggles.
- Make your own indoor hovercraft as seen on Living Life and Learning.
- Learn about colors with this beautiful butterfly chromatography craft as seen on Buggy and Buddy.
- Make soap souffle as seen on Steve Spangler Science.
- After talking about liquids and solids (and finding them in your own home), create oobleck as seen on Babble Dabble Do. Is it a liquid, or is it a solid?
- Learn about frost by making some indoor frost as seen on Little Bin for Little Hands.
- Make your own homemade butter in a jar as seen on Happy Hooligans.
What scientific experiment will you try first?

About Brigitte Brulz
Brigitte Brulz is a homeschooling mom of two daughters, wife of her high school sweetheart, and author of Jobs of a Preschooler and Pickles, Pickles, I Like Pickles. She offers free coloring pages and activity ideas on her website at BrigitteBrulz.com .
More Hands on Kids Activities to Try

Reader Interactions
30 comments.
college brawl says
March 13, 2024 at 1:05 am
Wow, these experiments look like so much fun! I can’t wait to try them out with my kids. We’re always looking for new and creative ways to learn about science at home, and these experiments look like they’ll be perfect for us. Thanks for sharing! 😊
threadsBay says
August 31, 2023 at 3:13 am
I love science experiments! This one is really simple and easy to do.
Leave a Comment Cancel reply
Your email address will not be published. Required fields are marked *
This site uses Akismet to reduce spam. Learn how your comment data is processed .

What Parents Have to Say…
Shop ebooks of activities.

Get activity plans delivered to your inbox, every week!
Activities that hands-on parents absolutely love.

6 Different Activities for 6 Lines of Tape

50 Simple & Fun Alphabet Activities for Preschoolers

How to Make a Lava Lamp Experiment Without Alka Seltzer

40+ Awesome Number Activities for Preschoolers

Top Physical Activities for Toddlers! Mom, Embrace the Energy!

Improve Kids Fine Motor Skills with 30 Materials & Activities
Get started having fun with your kids.
PLAN THE FUN WITH THE FREE KIDS ACTIVITIES PLANNER! AND RECEIVE ACTIVITIES EVERY WEEK!
- Preschoolers
- Kindergartners
- Grade School
- Literacy & ABCs
- Math & 123s
- Art Projects
- Gross Motor
- Shop Activity Plans
- Member Login

Choose Your Test
- Search Blogs By Category
- College Admissions
- AP and IB Exams
- GPA and Coursework
37 Cool Science Experiments for Kids to Do at Home
General Education

Are you looking for cool science experiments for kids at home or for class? We've got you covered! We've compiled a list of 37 of the best science experiments for kids that cover areas of science ranging from outer space to dinosaurs to chemical reactions. By doing these easy science experiments, kids will make their own blubber and see how polar bears stay warm, make a rain cloud in a jar to observe how weather changes, create a potato battery that'll really power a lightbulb, and more.
Below are 37 of the best science projects for kids to try. For each one we include a description of the experiment, which area(s) of science it teaches kids about, how difficult it is (easy/medium/hard), how messy it is (low/medium/high), and the materials you need to do the project. Note that experiments labelled "hard" are definitely still doable; they just require more materials or time than most of these other science experiments for kids.
#1: Insect Hotels
- Teaches Kids About: Zoology
- Difficulty Level: Medium
- Messiness Level: Medium
Insect hotels can be as simple (just a few sticks wrapped in a bundle) or as elaborate as you'd like, and they're a great way for kids to get creative making the hotel and then get rewarded by seeing who has moved into the home they built. After creating a hotel with hiding places for bugs, place it outside (near a garden is often a good spot), wait a few days, then check it to see who has occupied the "rooms." You can also use a bug ID book or app to try and identify the visitors.
- Materials Needed
- Shadow box or other box with multiple compartments
- Hot glue gun with glue
- Sticks, bark, small rocks, dried leaves, bits of yarn/wool, etc.

#2: DIY Lava Lamp
- Teaches Kids About: Chemical reactions
- Difficulty Level: Easy
In this quick and fun science experiment, kids will mix water, oil, food coloring, and antacid tablets to create their own (temporary) lava lamp . Oil and water don't mix easily, and the antacid tablets will cause the oil to form little globules that are dyed by the food coloring. Just add the ingredients together and you'll end up with a homemade lava lamp!
- Vegetable oil
- Food coloring
- Antacid tablets
#3: Magnetic Slime
- Teaches Kids About: Magnets
- Messiness Level: High (The slime is black and will slightly dye your fingers when you play with it, but it washes off easily.)
A step up from silly putty and Play-Doh, magnetic slime is fun to play with but also teaches kids about magnets and how they attract and repel each other. Some of the ingredients you aren't likely to have around the house, but they can all be purchased online. After mixing the ingredients together, you can use the neodymium magnet (regular magnets won't be strong enough) to make the magnetic slime move without touching it!
- Liquid starch
- Adhesive glue
- Iron oxide powder
- Neodymium (rare earth) magnet
#4: Baking Soda Volcanoes
- Teaches Kids About: Chemical reactions, earth science
- Difficulty Level: Easy-medium
- Messiness Level: High
Baking soda volcanoes are one of the classic science projects for kids, and they're also one of the most popular. It's hard to top the excitement of a volcano erupting inside your home. This experiment can also be as simple or in-depth as you like. For the eruption, all you need is baking soda and vinegar (dishwashing detergent adds some extra power to the eruption), but you can make the "volcano" as elaborate and lifelike as you wish.
- Baking soda
- Dishwashing detergent
- Large mason jar or soda bottle
- Playdough or aluminum foil to make the "volcano"
- Additional items to place around the volcano (optional)
- Food coloring (optional)
#5: Tornado in a Jar
- Teaches Kids About: Weather
- Messiness Level: Low
This is one of the quick and easy and science experiments for kids to teach them about weather. It only takes about five minutes and a few materials to set up, but once you have it ready you and your kids can create your own miniature tornado whose vortex you can see and the strength of which you can change depending on how quickly you swirl the jar.
- Glitter (optional)
#6: Colored Celery Experiment
- Teaches Kids About: Plants
This celery science experiment is another classic science experiment that parents and teachers like because it's easy to do and gives kids a great visual understanding of how transpiration works and how plants get water and nutrients. Just place celery stalks in cups of colored water, wait at least a day, and you'll see the celery leaves take on the color of the water. This happens because celery stalks (like other plants) contain small capillaries that they use to transport water and nutrients throughout the plant.
- Celery stalks (can also use white flowers or pale-colored cabbage)
#7: Rain Cloud in a Jar
This experiment teaches kids about weather and lets them learn how clouds form by making their own rain cloud . This is definitely a science project that requires adult supervision since it uses boiling water as one of the ingredients, but once you pour the water into a glass jar, the experiment is fast and easy, and you'll be rewarded with a little cloud forming in the jar due to condensation.
- Glass jar with a lid
- Boiling water
- Aerosol hairspray

#8: Edible Rock Candy
- Teaches Kids About: Crystal formation
It takes about a week for the crystals of this rock candy experiment to form, but once they have you'll be able to eat the results! After creating a sugar solution, you'll fill jars with it and dangle strings in them that'll slowly become covered with the crystals. This experiment involves heating and pouring boiling water, so adult supervision is necessary, once that step is complete, even very young kids will be excited to watch crystals slowly form.
- Large saucepan
- Clothespins
- String or small skewers
- Candy flavoring (optional)
#9: Water Xylophone
- Teaches Kids About: Sound waves
With just some basic materials you can create your own musical instrument to teach kids about sound waves. In this water xylophone experiment , you'll fill glass jars with varying levels of water. Once they're all lined up, kids can hit the sides with wooden sticks and see how the itch differs depending on how much water is in the jar (more water=lower pitch, less water=higher pitch). This is because sound waves travel differently depending on how full the jars are with water.
- Wooden sticks/skewers
#10: Blood Model in a Jar
- Teaches Kids About: Human biology
This blood model experiment is a great way to get kids to visual what their blood looks like and how complicated it really is. Each ingredient represents a different component of blood (plasma, platelets, red blood cells, etc.), so you just add a certain amount of each to the jar, swirl it around a bit, and you have a model of what your blood looks like.
- Empty jar or bottle
- Red cinnamon candies
- Marshmallows or dry white lima beans
- White sprinkles
#11: Potato Battery
- Teaches Kids About: Electricity
- Difficulty Level: Hard
Did you know that a simple potato can produce enough energy to keep a light bulb lit for over a month? You can create a simple potato battery to show kids. There are kits that provide all the necessary materials and how to set it up, but if you don't purchase one of these it can be a bit trickier to gather everything you need and assemble it correctly. Once it's set though, you'll have your own farm grown battery!
- Fresh potato
- Galvanized nail
- Copper coin

#12: Homemade Pulley
- Teaches Kids About: Simple machines
This science activity requires some materials you may not already have, but once you've gotten them, the homemade pulley takes only a few minutes to set up, and you can leave the pulley up for your kids to play with all year round. This pulley is best set up outside, but can also be done indoors.
- Clothesline
- 2 clothesline pulleys
#13: Light Refraction
- Teaches Kids About: Light
This light refraction experiment takes only a few minutes to set up and uses basic materials, but it's a great way to show kids how light travels. You'll draw two arrows on a sticky note, stick it to the wall, then fill a clear water bottle with water. As you move the water bottle in front of the arrows, the arrows will appear to change the direction they're pointing. This is because of the refraction that occurs when light passes through materials like water and plastic.
- Sticky note
- Transparent water bottle
#14: Nature Journaling
- Teaches Kids About: Ecology, scientific observation
A nature journal is a great way to encourage kids to be creative and really pay attention to what's going on around them. All you need is a blank journal (you can buy one or make your own) along with something to write with. Then just go outside and encourage your children to write or draw what they notice. This could include descriptions of animals they see, tracings of leaves, a drawing of a beautiful flower, etc. Encourage your kids to ask questions about what they observe (Why do birds need to build nests? Why is this flower so brightly colored?) and explain to them that scientists collect research by doing exactly what they're doing now.
- Blank journal or notebook
- Pens/pencils/crayons/markers
- Tape or glue for adding items to the journal
#15: DIY Solar Oven
- Teaches Kids About: Solar energy
This homemade solar oven definitely requires some adult help to set up, but after it's ready you'll have your own mini oven that uses energy from the sun to make s'mores or melt cheese on pizza. While the food is cooking, you can explain to kids how the oven uses the sun's rays to heat the food.
- Aluminum foil
- Knife or box cutter
- Permanent marker
- Plastic cling wrap
- Black construction paper

#16: Animal Blubber Simulation
- Teaches Kids About: Ecology, zoology
If your kids are curious about how animals like polar bears and seals stay warm in polar climates, you can go beyond just explaining it to them; you can actually have them make some of their own blubber and test it out. After you've filled up a large bowl with ice water and let it sit for a few minutes to get really cold, have your kids dip a bare hand in and see how many seconds they can last before their hand gets too cold. Next, coat one of their fingers in shortening and repeat the experiment. Your child will notice that, with the shortening acting like a protective layer of blubber, they don't feel the cold water nearly as much.
- Bowl of ice water
#17: Static Electricity Butterfly
This experiment is a great way for young kids to learn about static electricity, and it's more fun and visual than just having them rub balloons against their heads. First you'll create a butterfly, using thick paper (such as cardstock) for the body and tissue paper for the wings. Then, blow up the balloon, have the kids rub it against their head for a few seconds, then move the balloon to just above the butterfly's wings. The wings will move towards the balloon due to static electricity, and it'll look like the butterfly is flying.
- Tissue paper
- Thick paper
- Glue stick/glue
#18: Edible Double Helix
- Teaches Kids About: Genetics
If your kids are learning about genetics, you can do this edible double helix craft to show them how DNA is formed, what its different parts are, and what it looks like. The licorice will form the sides or backbone of the DNA and each color of marshmallow will represent one of the four chemical bases. Kids will be able to see that only certain chemical bases pair with each other.
- 2 pieces of licorice
- 12 toothpicks
- Small marshmallows in 4 colors (9 of each color)
- 5 paperclips
#19: Leak-Proof Bag
- Teaches Kids About: Molecules, plastics
This is an easy experiment that'll appeal to kids of a variety of ages. Just take a zip-lock bag, fill it about ⅔ of the way with water, and close the top. Next, poke a few sharp objects (like bamboo skewers or sharp pencils) through one end and out the other. At this point you may want to dangle the bag above your child's head, but no need to worry about spills because the bag won't leak? Why not? It's because the plastic used to make zip-lock bags is made of polymers, or long chains of molecules that'll quickly join back together when they're forced apart.
- Zip-lock bags
- Objects with sharp ends (pencils, bamboo skewers, etc.)

#20: How Do Leaves Breathe?
- Teaches Kids About: Plant science
It takes a few hours to see the results of this leaf experiment , but it couldn't be easier to set up, and kids will love to see a leaf actually "breathing." Just get a large-ish leaf, place it in a bowl (glass works best so you can see everything) filled with water, place a small rock on the leaf to weigh it down, and leave it somewhere sunny. Come back in a few hours and you'll see little bubbles in the water created when the leaf releases the oxygen it created during photosynthesis.
- Large bowl (preferably glass)
- Magnifying glass (optional)
#21: Popsicle Stick Catapults
Kids will love shooting pom poms out of these homemade popsicle stick catapults . After assembling the catapults out of popsicle sticks, rubber bands, and plastic spoons, they're ready to launch pom poms or other lightweight objects. To teach kids about simple machines, you can ask them about how they think the catapults work, what they should do to make the pom poms go a farther/shorter distance, and how the catapult could be made more powerful.
- Popsicle sticks
- Rubber bands
- Plastic spoons
- Paint (optional)
#22: Elephant Toothpaste
You won't want to do this experiment near anything that's difficult to clean (outside may be best), but kids will love seeing this " elephant toothpaste " crazily overflowing the bottle and oozing everywhere. Pour the hydrogen peroxide, food coloring, and dishwashing soap into the bottle, and in the cup mix the yeast packet with some warm water for about 30 seconds. Then, add the yeast mixture to the bottle, stand back, and watch the solution become a massive foamy mixture that pours out of the bottle! The "toothpaste" is formed when the yeast removed the oxygen bubbles from the hydrogen peroxide which created foam. This is an exothermic reaction, and it creates heat as well as foam (you can have kids notice that the bottle became warm as the reaction occurred).
- Clean 16-oz soda bottle
- 6% solution of hydrogen peroxide
- 1 packet of dry yeast
- Dishwashing soap
#23: How Do Penguins Stay Dry?
Penguins, and many other birds, have special oil-producing glands that coat their feathers with a protective layer that causes water to slide right off them, keeping them warm and dry. You can demonstrate this to kids with this penguin craft by having them color a picture of a penguin with crayons, then spraying the picture with water. The wax from the crayons will have created a protective layer like the oil actual birds coat themselves with, and the paper won't absorb the water.
- Penguin image (included in link)
- Spray bottle
- Blue food coloring (optional)

#24: Rock Weathering Experiment
- Teaches Kids About: Geology
This mechanical weathering experiment teaches kids why and how rocks break down or erode. Take two pieces of clay, form them into balls, and wrap them in plastic wrap. Then, leave one out while placing the other in the freezer overnight. The next day, unwrap and compare them. You can repeat freezing the one piece of clay every night for several days to see how much more cracked and weathered it gets than the piece of clay that wasn't frozen. It may even begin to crumble. This weathering also happens to rocks when they are subjected to extreme temperatures, and it's one of the causes of erosion.
- Plastic wrap
#25: Saltwater Density
- Teaches Kids About: Water density
For this saltwater density experiment , you'll fill four clear glasses with water, then add salt to one glass, sugar to one glass, and baking soda to one glass, leaving one glass with just water. Then, float small plastic pieces or grapes in each of the glasses and observe whether they float or not. Saltwater is denser than freshwater, which means some objects may float in saltwater that would sink in freshwater. You can use this experiment to teach kids about the ocean and other bodies of saltwater, such as the Dead Sea, which is so salty people can easily float on top of it.
- Four clear glasses
- Lightweight plastic objects or small grapes
#26: Starburst Rock Cycle
With just a package of Starbursts and a few other materials, you can create models of each of the three rock types: igneous, sedimentary, and metamorphic. Sedimentary "rocks" will be created by pressing thin layers of Starbursts together, metamorphic by heating and pressing Starbursts, and igneous by applying high levels of heat to the Starbursts. Kids will learn how different types of rocks are forms and how the three rock types look different from each other.
- Toaster oven
#27: Inertia Wagon Experiment
- Teaches Kids About: Inertia
This simple experiment teaches kids about inertia (as well as the importance of seatbelts!). Take a small wagon, fill it with a tall stack of books, then have one of your children pull it around then stop abruptly. They won't be able to suddenly stop the wagon without the stack of books falling. You can have the kids predict which direction they think the books will fall and explain that this happens because of inertia, or Newton's first law.
- Stack of books
#28: Dinosaur Tracks
- Teaches Kids About: Paleontology
How are some dinosaur tracks still visible millions of years later? By mixing together several ingredients, you'll get a claylike mixture you can press your hands/feet or dinosaur models into to make dinosaur track imprints . The mixture will harden and the imprints will remain, showing kids how dinosaur (and early human) tracks can stay in rock for such a long period of time.
- Used coffee grounds
- Wooden spoon
- Rolling pin
#29: Sidewalk Constellations
- Teaches Kids About: Astronomy
If you do this sidewalk constellation craft , you'll be able to see the Big Dipper and Orion's Belt in the daylight. On the sidewalk, have kids draw the lines of constellations (using constellation diagrams for guidance) and place stones where the stars are. You can then look at astronomy charts to see where the constellations they drew will be in the sky.
- Sidewalk chalk
- Small stones
- Diagrams of constellations
#30: Lung Model
By building a lung model , you can teach kids about respiration and how their lungs work. After cutting off the bottom of a plastic bottle, you'll stretch a balloon around the opened end and insert another balloon through the mouth of the bottle. You'll then push a straw through the neck of the bottle and secure it with a rubber band and play dough. By blowing into the straw, the balloons will inflate then deflate, similar to how our lungs work.
- Plastic bottle
- Rubber band

#31: Homemade Dinosaur Bones
By mixing just flour, salt, and water, you'll create a basic salt dough that'll harden when baked. You can use this dough to make homemade dinosaur bones and teach kids about paleontology. You can use books or diagrams to learn how different dinosaur bones were shaped, and you can even bury the bones in a sandpit or something similar and then excavate them the way real paleontologists do.
- Images of dinosaur bones
#32: Clay and Toothpick Molecules
There are many variations on homemade molecule science crafts . This one uses clay and toothpicks, although gumdrops or even small pieces of fruit like grapes can be used in place of clay. Roll the clay into balls and use molecule diagrams to attach the clay to toothpicks in the shape of the molecules. Kids can make numerous types of molecules and learn how atoms bond together to form molecules.
- Clay or gumdrops (in four colors)
- Diagrams of molecules
#33: Articulated Hand Model
By creating an articulated hand model , you can teach kids about bones, joints, and how our hands are able to move in many ways and accomplish so many different tasks. After creating a hand out of thin foam, kids will cut straws to represent the different bones in the hand and glue them to the fingers of the hand models. You'll then thread yarn (which represents tendons) through the straws, stabilize the model with a chopstick or other small stick, and end up with a hand model that moves and bends the way actual human hands do.
- Straws (paper work best)
- Twine or yarn
#34: Solar Energy Experiment
- Teaches Kids About: Solar energy, light rays
This solar energy science experiment will teach kids about solar energy and how different colors absorb different amounts of energy. In a sunny spot outside, place six colored pieces of paper next to each other, and place an ice cube in the middle of each paper. Then, observe how quickly each of the ice cubes melt. The ice cube on the black piece of paper will melt fastest since black absorbs the most light (all the light ray colors), while the ice cube on the white paper will melt slowest since white absorbs the least light (it instead reflects light). You can then explain why certain colors look the way they do. (Colors besides black and white absorb all light except for the one ray color they reflect; this is the color they appear to us.)
- 6 squares of differently colored paper/cardstock (must include black paper and white paper)
#35: How to Make Lightning
- Teaches Kids About: Electricity, weather
You don't need a storm to see lightning; you can actually create your own lightning at home . For younger kids this experiment requires adult help and supervision. You'll stick a thumbtack through the bottom of an aluminum tray, then stick the pencil eraser to the pushpin. You'll then rub the piece of wool over the aluminum tray, and then set the tray on the Styrofoam, where it'll create a small spark/tiny bolt of lightning!
- Pencil with eraser
- Aluminum tray or pie tin
- Styrofoam tray
#36: Tie-Dyed Milk
- Teaches Kids About: Surface tension
For this magic milk experiment , partly fill a shallow dish with milk, then add a one drop of each food coloring color to different parts of the milk. The food coloring will mostly stay where you placed it. Next, carefully add one drop of dish soap to the middle of the milk. It'll cause the food coloring to stream through the milk and away from the dish soap. This is because the dish soap breaks up the surface tension of the milk by dissolving the milk's fat molecules.
- Shallow dish
- Milk (high-fat works best)

#37: How Do Stalactites Form?
Have you ever gone into a cave and seen huge stalactites hanging from the top of the cave? Stalactites are formed by dripping water. The water is filled with particles which slowly accumulate and harden over the years, forming stalactites. You can recreate that process with this stalactite experiment . By mixing a baking soda solution, dipping a piece of wool yarn in the jar and running it to another jar, you'll be able to observe baking soda particles forming and hardening along the yarn, similar to how stalactites grow.
- Safety pins
- 2 glass jars
Summary: Cool Science Experiments for Kids
Any one of these simple science experiments for kids can get children learning and excited about science. You can choose a science experiment based on your child's specific interest or what they're currently learning about, or you can do an experiment on an entirely new topic to expand their learning and teach them about a new area of science. From easy science experiments for kids to the more challenging ones, these will all help kids have fun and learn more about science.
What's Next?
Are you also interested in pipe cleaner crafts for kids? We have a guide to some of the best pipe cleaner crafts to try!
Looking for multiple different slime recipes? We tell you how to make slimes without borax and without glue as well as how to craft the ultimate super slime .
Want to learn more about clouds? Learn how to identify every cloud in the sky with our guide to the 10 types of clouds .
Want to know the fastest and easiest ways to convert between Fahrenheit and Celsius? We've got you covered! Check out our guide to the best ways to convert Celsius to Fahrenheit (or vice versa) .
Trending Now
How to Get Into Harvard and the Ivy League
How to Get a Perfect 4.0 GPA
How to Write an Amazing College Essay
What Exactly Are Colleges Looking For?
ACT vs. SAT: Which Test Should You Take?
When should you take the SAT or ACT?
Get Your Free

Find Your Target SAT Score
Free Complete Official SAT Practice Tests
How to Get a Perfect SAT Score, by an Expert Full Scorer
Score 800 on SAT Math
Score 800 on SAT Reading and Writing
How to Improve Your Low SAT Score
Score 600 on SAT Math
Score 600 on SAT Reading and Writing
Find Your Target ACT Score
Complete Official Free ACT Practice Tests
How to Get a Perfect ACT Score, by a 36 Full Scorer
Get a 36 on ACT English
Get a 36 on ACT Math
Get a 36 on ACT Reading
Get a 36 on ACT Science
How to Improve Your Low ACT Score
Get a 24 on ACT English
Get a 24 on ACT Math
Get a 24 on ACT Reading
Get a 24 on ACT Science
Stay Informed
Get the latest articles and test prep tips!

Christine graduated from Michigan State University with degrees in Environmental Biology and Geography and received her Master's from Duke University. In high school she scored in the 99th percentile on the SAT and was named a National Merit Finalist. She has taught English and biology in several countries.
Ask a Question Below
Have any questions about this article or other topics? Ask below and we'll reply!
Science Bob
- Experiments
- Science Fair Ideas
- Science Q&A
- Research Help
- Experiment Blog
Okay, this is the hardest part of the whole project…picking your topic. But here are some ideas to get you started. Even if you don’t like any, they may inspire you to come up with one of your own. Remember, check all project ideas with your teacher and parents, and don’t do any project that would hurt or scare people or animals. Good luck!
- Does music affect on animal behavior?
- Does the color of food or drinks affect whether or not we like them?
- Where are the most germs in your school? ( CLICK for more info. )
- Does music have an affect on plant growth?
- Which kind of food do dogs (or any animal) prefer best?
- Which paper towel brand is the strongest?
- What is the best way to keep an ice cube from melting?
- What level of salt works best to hatch brine shrimp?
- Can the food we eat affect our heart rate?
- How effective are child-proof containers and locks.
- Can background noise levels affect how well we concentrate?
- Does acid rain affect the growth of aquatic plants?
- What is the best way to keep cut flowers fresh the longest?
- Does the color of light used on plants affect how well they grow?
- What plant fertilizer works best?
- Does the color of a room affect human behavior?
- Do athletic students have better lung capacity?
- What brand of battery lasts the longest?
- Does the type of potting soil used in planting affect how fast the plant grows?
- What type of food allow mold to grow the fastest?
- Does having worms in soil help plants grow faster?
- Can plants grow in pots if they are sideways or upside down?
- Does the color of hair affect how much static electricity it can carry? (test with balloons)
- How much weight can the surface tension of water hold?
- Can some people really read someone else’s thoughts?
- Which soda decays fallen out teeth the most?
- What light brightness makes plants grow the best?
- Does the color of birdseed affect how much birds will eat it?
- Do natural or chemical fertilizers work best?
- Can mice learn? (you can pick any animal)
- Can people tell artificial smells from real ones?
- What brands of bubble gum produce the biggest bubbles?
- Does age affect human reaction times?
- What is the effect of salt on the boiling temperature of water?
- Does shoe design really affect an athlete’s jumping height?
- What type of grass seed grows the fastest?
- Can animals see in the dark better than humans?
Didn’t see one you like? Don’t worry…look over them again and see if they give you an idea for your own project that will work for you. Remember, find something that interests you, and have fun with it.
To download and print this list of ideas CLICK HERE .

- The scientific method
- science fair resources
- a little helpful advice
ADS (these ads support our free website)
Share this page.
The Top Ten Scientific Discoveries of the Decade
Breakthroughs include measuring the true nature of the universe, finding new species of human ancestors, and unlocking new ways to fight disease
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/bennett.jpg)
Jay Bennett
Former associate web editor, science.
:focal(800x601:801x602)/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/d4/a8/d4a88985-6b69-41f0-85b3-4425e6c98596/science_rules5.jpg)
Millions of new scientific research papers are published every year , shedding light on everything from the evolution of stars to the ongoing impacts of climate change to the health benefits (or determents) of coffee to the tendency of your cat to ignore you . With so much research coming out every year, it can be difficult to know what is significant, what is interesting but largely insignificant, and what is just plain bad science . But over the course of a decade, we can look back at some of the most important and awe-inspiring areas of research, often expressed in multiple findings and research papers that lead to a true proliferation of knowledge. Here are ten of the biggest strides made by scientists in the last ten years.
New Human Relatives
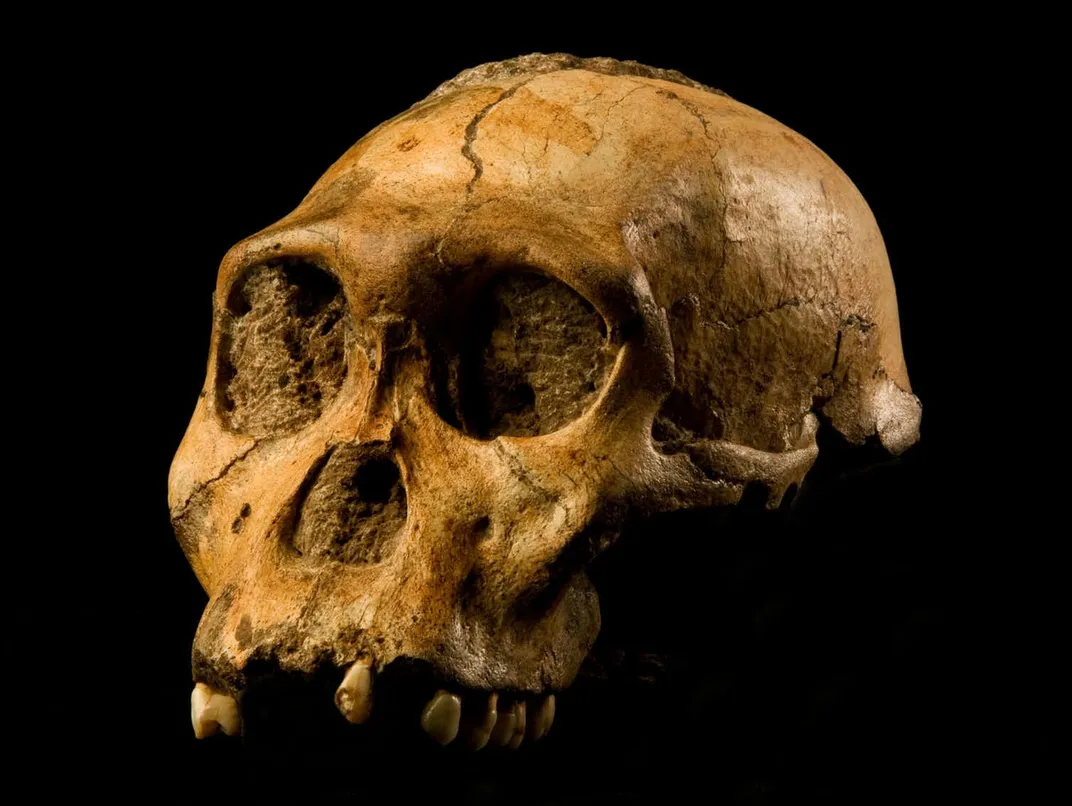
The human family tree expanded significantly in the past decade, with fossils of new hominin species discovered in Africa and the Philippines. The decade began with the discovery and identification of Australopithecus sediba , a hominin species that lived nearly two million years ago in present-day South Africa. Matthew Berger, the son of paleoanthropologist Lee Berger, stumbled upon the first fossil of the species, a right clavicle, in 2008, when he was only 9 years old. A team then unearthed more fossils from the individual, a young boy, including a well-preserved skull, and A. sediba was described by Lee Berger and colleagues in 2010 . The species represents a transitionary phase between the genus Australopithecus and the genus Homo , with some traits of the older primate group but a style of walking that resembled modern humans.
Also discovered in South Africa by a team led by Berger, Homo naledi lived much more recently, some 335,000 to 236,000 years ago, meaning it may have overlapped with our own species, Homo sapiens. The species, first discovered in the Rising Star Cave system in 2013 and described in 2015 , also had a mix of primitive and modern features, such as a small brain case (about one-third the size of Homo sapiens ) and a large body for the time, weighing approximately 100 pounds and standing up to five feet tall. The smaller Homo luzonensis (three to four feet tall) lived in the Philippines some 50,000 to 67,000 years ago , overlapping with several species of hominin. The first H. luzonensis fossils were originally identified as Homo sapiens, but a 2019 analysis determined that the bones belonged to an entirely unknown species.
These three major finds in the last ten years suggest that the bones of more species of ancient human relatives are likely hidden in the caves and sediment deposits of the world, waiting to be discovered.
Taking Measure of the Cosmos

When Albert Einstein first published the general theory of relativity in 1915, he likely couldn’t have imagined that 100 years later, astronomers would test the theory’s predictions with some of the most sophisticated instruments ever built—and the theory would pass each test. General relativity describes the universe as a “fabric” of space-time that is warped by large masses. It’s this warping that causes gravity, rather than an internal property of mass as Isaac Newton thought.
One prediction of this model is that the acceleration of masses can cause “ripples” in space-time, or the propagation of gravitational waves. With a large enough mass, such as a black hole or a neutron star, these ripples may even be detected by astronomers on Earth. In September 2015, the LIGO and Virgo collaboration detected gravitational waves for the first time, propagating from a pair of merging black holes some 1.3 billion light-years away. Since then, the two instruments have detected several additional gravitational waves , including one from a two merging neutron stars.
Another prediction of general relativity—one that Einstein himself famously doubted —is the existence of black holes at all, or points of gravitational collapse in space with infinite density and infinitesimal volume. These objects consume all matter and light that strays too close, creating a disk of superheated material falling into the black hole. In 2017, the Event Horizon Telescope collaboration —a network of linked radio telescopes around the world—took observations that would later result in the first image of the environment around a black hole, released in April 2019 .
The Hottest Years on Record

Scientists have been predicating the effects of burning coal and fossil fuels on the temperature of the planet for over 100 years. A 1912 issue of Popular Mechanics contains an article titled “ Remarkable Weather of 1911: The Effect of the Combustion of Coal on the Climate—What Scientists Predict for the Future ,” which has a caption that reads: “The furnaces of the world are now burning about 2,000,000,000 tons of coal a year. When this is burned, uniting with oxygen, it adds about 7,000,000,000 tons of carbon dioxide to the atmosphere yearly. This tends to make the air a more effective blanket for the earth and to raise its temperature. The effect may be considerable in a few centuries.”
Just one century later, and the effect is considerable indeed. Increased greenhouse gases in the atmosphere have produced hotter global temperatures, with the last five years (2014 to 2018) being the hottest years on record . 2016 was the hottest year since the National Oceanic and Atmospheric Administration (NOAA) started recording global temperature 139 years ago. The effects of this global change include more frequent and destructive wildfires, more common droughts, accelerating polar ice melt and increased storm surges. California is burning, Venice is flooding, urban heat deaths are on the rise, and countless coastal and island communities face an existential crisis—not to mention the ecological havoc wreaked by climate change, stifling the planet’s ability to pull carbon back out of the atmosphere.
In 2015, the United Nations Framework Convention on Climate Change (UNFCCC) reached a consensus on climate action, known as the Paris Agreement. The primary goal of the Paris Agreement is to limit global temperature increases to 1.5 degrees Celsius over pre-industrial levels . To achieve this goal, major societal transformations will be required, including replacing fossil fuels with clean energy such as wind, solar and nuclear; reforming agricultural practices to limit emissions and protect forested areas; and perhaps even building artificial means of pulling carbon dioxide out of the atmosphere.
Editing Genes

Ever since the double-helix structure of DNA was revealed in the early 1950s , scientists have hypothesized about the possibility of artificially modifying DNA to change the functions of an organism. The first approved gene therapy trial occurred in 1990, when a four-year-old girl had her own white blood cells removed, augmented with the genes that produce an enzyme called adenosine deaminase (ADA), and then reinjected into her body to treat ADA deficiency, a genetic condition that hampers the immune system’s ability to fight disease. The patient’s body began producing the ADA enzyme, but new white blood cells with the corrected gene were not produced, and she had to continue receiving injections .
Now, genetic engineering is more precise and available than ever before, thanks in large part to a new tool first used to modify eukaryotic cells (complex cells with a nucleus) in 2013 : CRISPR-Cas9. The gene editing tool works by locating a targeted section of DNA and “cutting” out that section with the Cas9 enzyme. An optional third step involves replacing the deleted section of DNA with new genetic material. The technique can be used for a wide range of applications, from increasing the muscle mass of livestock, to producing resistant and fruitful crops, to treating diseases like cancer by removing a patient’s immune system cells, modifying them to better fight a disease, and reinjecting them into the patient’s body.
In late 2018, Chinese researchers led by He Jiankui announced that they had used CRISPR-Cas9 to genetically modify human embryos, which were then transferred to a woman’s uterus and resulted in the birth of twin girls—the first gene-edited babies. The twins’ genomes were modified to make the girls more resistant to HIV, although the genetic alterations may have also resulted in unintended changes . The work was widely condemned by the scientific community as unethical and dangerous, revealing a need for stricter regulations for how these powerful new tools are used, particularly when it comes to changing the DNA of embryos and using those embryos to birth live children.
Mysteries of Other Worlds Revealed

Spacecraft and telescopes have revealed a wealth of information about worlds beyond our own in the last decade. In 2015, the New Horizons probe made a close pass of Pluto, taking the first nearby observations of the dwarf planet and its moons. The spacecraft revealed a surprisingly dynamic and active world, with icy mountains reaching up to nearly 20,000 feet and shifting plains that are no more than 10 million years old—meaning the geology is constantly changing. The fact that Pluto—which is an average of 3.7 billion miles from the sun , about 40 times the distance of Earth—is so geologically active suggests that even cold, distant worlds could get enough energy to heat their interiors, possibly harboring subsurface liquid water or even life.
A bit closer to home, the Cassini spacecraft orbited Saturn for 13 years , ending its mission in September 2017 when NASA intentionally plunged the spacecraft into the atmosphere of Saturn so it would burn up rather than continue orbiting the planet once it had exhausted its fuel. During its mission, Cassini discovered the processes that feed Saturn’s rings , observed a global storm encircle the gas giant, mapped the large moon Titan and found some of the ingredients for life in the plumes of icy material erupting from the watery moon Enceladus. In 2016, a year before the end of the Cassini mission, the Juno spacecraft arrived at Jupiter, where it has been measuring the magnetic field and atmospheric dynamics of the largest planet in the solar system to help scientists understand how Jupiter—and everything else around the sun—originally formed.
In 2012, the Curiosity rover landed on Mars, where it has made several significant discoveries, including new evidence of past water on the red planet , the presence of organic molecules that could be related to life, and mysterious seasonal cycles of methane and oxygen that hint at a dynamic world beneath the surface. In 2018, the European Space Agency announced that ground-penetrating radar data from the Mars Express spacecraft provided strong evidence that a liquid reservoir of water exists underground near the Martian south pole .
Meanwhile, two space telescopes, Kepler and TESS, have discovered thousands of planets orbiting other stars. Kepler launched in 2009 and ended its mission in 2018, revealing mysterious and distant planets by measuring the decrease in light when they pass in front of their stars. These planets include hot Jupiters, which orbit close to their stars in just days or hours; mini Neptunes, which are between the size of Earth and Neptune and may be gas, liquid, solid or some combination; and super Earths, which are large rocky planets that astronomers hope to study for signs of life. TESS, which launched in 2018, continues the search as Kepler’s successor. The space telescope has already discovered hundreds of worlds , and it could find 10,000 or even 20,000 before the end of the mission.
Fossilized Pigments Reveal the Colors of Dinosaurs

The decade began with a revolution in paleontology as scientists got their first look at the true colors of dinosaurs. First, in January 2010, an analysis of melanosomes—organelles that contain pigments—in the fossilized feathers of Sinosauropteryx , a dinosaur that lived in China some 120 to 125 million years ago, revealed that the prehistoric creature had “reddish-brown tones” and stripes along its tail . Shortly after, a full-body reconstruction revealed the colors of a small feathered dinosaur that lived some 160 million years ago , Anchiornis , which had black and white feathers on its body and a striking plume of red feathers on its head.
The study of fossilized pigments has continued to expose new information about prehistoric life, hinting at potential animal survival strategies by showing evidence of countershading and camouflage . In 2017, a remarkably well-preserved armored dinosaur which lived about 110 million years ago, Borealopelta , was found to have reddish-brown tones to help blend into the environment . This new ability to identify and study the colors of dinosaurs will continue to play an important role in paleontological research as scientists study the evolution of past life.
Redefining the Fundamental Unit of Mass
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/4d/4f/4d4f416e-6e5d-41fe-b6f7-3a79044a0aab/wattbalance_rsi_cover-1.jpg)
In November 2018, measurement scientists around the world voted to officially changed the definition of a kilogram , the fundamental unit of mass. Rather than basing the kilogram off of an object—a platinum-iridium alloy cylinder about the size of a golf ball—the new definition uses a constant of nature to set the unit of mass. The change replaced the last physical artifact used to define a unit of measure. (The meter bar was replaced in 1960 by a specific number of wavelengths of radiation from krypton, for example, and later updated to define a meter according to the distance light travels in a tiny fraction of a second .)
By using a sophisticated weighing machine known as a Kibble balance, scientists were able to precisely measure a kilogram according to the electromagnetic force required to hold it up. This electric measurement could then be expressed in terms of Planck’s constant, a number originally used by Max Planck to calculate bundles of energy coming from stars .
The kilogram was not the only unit of measure that was recently redefined. The changes to the International System of Units, which officially went into effect in May 2019 , also changed the definition for the ampere, the standard unit of electric current; the kelvin unit of temperature; and the mole, a unit of amount of substance used in chemistry. The changes to the kilogram and other units will allow more precise measurements for small amounts of material, such as pharmaceuticals, as well as give scientists around the world access to the fundamental units, rather than defining them according to objects that must be replicated and calibrated by a small number of labs.
First Ancient Human Genome Sequenced

In 2010, scientists gained a new tool to study the ancient past and the people who inhabited it. Researchers used a hair preserved in permafrost to sequence the genome of a man who lived some 4,000 years ago in what is now Greenland , revealing the physical traits and even the blood type of a member of one of the first cultures to settle in that part of the world. The first nearly complete reconstruction of a genome from ancient DNA opened the door for anthropologists and geneticists to learn more about the cultures of the distant past than ever before.
Extracting ancient DNA is a major challenge. Even if genetic material such as hair or skin is preserved, it is often contaminated with the DNA of microbes from the environment, so sophisticated sequencing techniques must be used to isolate the ancient human’s DNA. More recently, scientists have used the petrous bone of the skull , a highly dense bone near the ear, to extract ancient DNA.
Thousands of ancient human genomes have been sequenced since the first success in 2010, revealing new details about the rise and fall of lost civilizations and the migrations of people around the globe . Studying ancient genomes has identified multiple waves of migration back and forth across the frozen Bering land bridge between Siberia and Alaska between 5,000 and 15,000 years ago. Recently, the genome of a young girl in modern Denmark was sequenced from a 5,700-year-old piece of birch tar used as chewing gum, which also contained her mouth microbes and bits of food from one of her last meals.
A Vaccine and New Treatments to Fight Ebola
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/filer/94/69/946983e3-aa54-4eeb-add1-1eea8092071c/gettyimages-1183891526.jpg)
This decade included the worst outbreak of Ebola virus diseases in history. The epidemic is believed to have begun with a single case of an 18-month-old-boy in Guinea infected by bats in December 2013. The disease quickly spread to neighboring countries, reaching the capitals of Liberia and Sierra Leone by July 2014, providing an unprecedented opportunity for the transmission of the disease to a large number of people. Ebola virus compromises the immune system and can cause massive hemorrhaging and multiple organ failure. Two and a half years after the initial case, more than 28,600 people had been infected, resulting in at least 11,325 deaths, according to the CDC .
The epidemic prompted health officials to redouble their efforts to find an effective vaccine to fight Ebola. A vaccine known as Ervebo, made by the pharmaceutical company Merck, was tested in a clinical trial in Guinea performed toward the end of the outbreak in 2016 that proved the vaccine effective. Another Ebola outbreak was declared in the Democratic Republic of the Congo in August 2018, and the ongoing epidemic has spread to become the deadliest since the West Africa outbreak, with 3,366 reported cases and 2,227 deaths as of December 2019 . Ervebo has been used in the DRC to fight the outbreak on an expanded access or “compassionate use” basis . In November 2019, Ervebo was approved by the European Medicines Agency (EMA) , and a month later it was approved in the U.S. by the FDA .
In addition to a preventative vaccine, researchers have been seeking a cure for Ebola in patients who have already been infected by the disease. Two treatments, which involve a one-time delivery of antibodies to prevent Ebola from infecting a patient’s cells, have recently shown promise in a clinical trial in the DRC . With a combination of vaccines and therapeutic treatments, healthcare officials hope to one day eradicate the viral infection for good .
CERN Detects the Higgs Boson
Over the past several decades, physicists have worked tirelessly to model the workings of the universe, developing what is known as the Standard Model. This model describes four basic interactions of matter, known as the fundamental forces . Two are familiar in everyday life: the gravitational force and the electromagnetic force. The other two, however, only exert their influence inside the nuclei of atoms: the strong nuclear force and the weak nuclear force.
Part of the Standard Model says that there is a universal quantum field that interacts with particles, giving them their masses. In the 1960s, theoretical physicists including François Englert and Peter Higgs described this field and its role in the Standard Model. It became known as the Higgs field, and according to the laws of quantum mechanics, all such fundamental fields should have an associated particle, which came to be known as the Higgs boson.
Decades later, in 2012, two teams using the Large Hadron Collider at CERN to conduct particle collisions reported the detection of a particle with the predicted mass of the Higgs boson, providing substantial evidence for the existence of the Higgs field and Higgs boson. In 2013, the Nobel Prize in Physics was awarded to Englert and Higgs “for the theoretical discovery of a mechanism that contributes to our understanding of the origin of mass of subatomic particles, and which recently was confirmed through the discovery of the predicted fundamental particle.” As physicists continue to refine the Standard Model, the function and discovery of the Higgs boson will remain a fundamental part of how all matter gets its mass, and therefore, how any matter exists at all.
Get the latest Science stories in your inbox.
/https://tf-cmsv2-smithsonianmag-media.s3.amazonaws.com/accounts/headshot/bennett.jpg)
Jay Bennett | | READ MORE
Jay Bennett was the associate web editor, science, for Smithsonian .
45 Easy Science Experiments for Kids
Hello, STEM! These simple DIY activities can be done at home or in school.

We've been independently researching and testing products for over 120 years. If you buy through our links, we may earn a commission. Learn more about our review process.
Imagine blowing the biggest bubbles imaginable — or even making bubbles within bubbles. Or sending vessels — rockets, tea bags, airplanes — soaring through the sky for impossible distances. Now imagine making things explode, or change colors, or reveal hidden messages with just a few simple mixtures.
None of this is magic. It's all science that you can do at home, most likely with ingredients you already have in your house. So, next time you need a boredom-busting indoor activity on a rainy day or a DIY project to get their minds humming, try one of these best at-home science experiments for kids , which cover topics like cover magnetism, surface tension, astronomy, chemistry, physics and more.
First off, it's good to start them off with the scientific method. Give them a journal to record their observations, questions, hypotheses, experiments, results and conclusions. As always, safety counts: wear goggles and coats or aprons if need be (sometimes kids get a kick out of how scientific the protective gear makes them look), and always make sure that the kids are supervised when doing them. (Warning: Some of these are messy!)
These experiments are mostly designed for preschoolers through elementary schoolers — with a couple that are either demonstrations or better for older kids — but if you have a younger one, you can check out these 1-year-old learning activities , toddler learning activities and preschool/kindergarten learning activities , some of which also cover STEM subjects.
Floating Fish

Here's another one that deals with solubility and density.
- Draw the outline of a fish on the bottom of a glass plate or tray in dry-erase marker. Retrace your drawing to make sure all the lines are connected. Let dry for a minute or two.
- Fill the measuring cup with tap water. Place the pour spout just inside the corner of the dish and add water very slowly until it just covers the bottom. Be careful not to pour water directly onto your drawing or make splashes near it. The water will move toward your drawing, eventually surrounding it. Observe what happens. If the water splashes or it doesn’t work on your first try, empty the dish, erase the drawing with a paper towel, dry off the dish, and try again.
- Tilt the dish slightly from side to side. What happens? Jot it down.
The ink in dry erase markers is engineered to be slippery. It’s made with a chemical that causes it to easily release from surfaces. (Permanent markers are made with a chemical that makes the ink stick to surfaces, so be sure not to use these in your experiment!)
The easy-release ink lets go from a surface, but why does it float? There are two reasons. First, dry erase ink isn’t soluble, which means it won’t dissolve in water. Second, dry erase ink is less dense than the water, so it becomes buoyant, meaning it can float. When you tilt the dish, the fish moves around on the water’s surface.
From Good Housekeeping Amazing Science: 83 Hands-on S.T.E.A.M Experiments for Curious Kids! See more in the book »
Brush, Brush!

This one will really get them into brushing their teeth once they scientifically prove all the good things that toothpaste can do.
- Write on sticky notes: Soda 1, Soda 2, Juice 1, and Juice 2. Place them in a row on a counter.
- Fill two glasses halfway with brown soda and place behind the Soda 1 and Soda 2 sticky notes. Fill two glasses halfway with lemon juice and place behind the Juice 1 and Juice 2 sticky notes.
- Carefully place one egg in the bowl. Squeeze a big dollop — about one tablespoon — of toothpaste on top of the egg and gently rub the toothpaste all around with your hands until the egg is completely covered in a thick layer of toothpaste. Repeat with a second egg.
- Gently submerge the toothpaste-covered eggs into the liquids: one egg in the glass labeled Soda 1 and the other egg in the glass labeled Juice 1. Wash and dry your hands.
- Gently submerge the remaining eggs, without toothpaste on them, in the remaining glasses: one in the glass labeled Soda 2 and the other in the glass of juice labeled Juice 2. Wash and dry your hands. Leave the eggs in the glasses for 12 hours.
- After 12 hours, remove the eggs from the glasses of soda one at a time. Rinse them in cool water and pat them dry with the towel. Place each egg by the sticky note of the glass it was in. Are the eggs the same or different colors?
- Remove the eggs from the glasses of juice one at a time. Rinse them under the faucet and pat them dry. Place each egg by the sticky note of the glass it was in. Feel the eggs gently. Does one feel stronger or weaker than the other?
- Write down your observations in your science notebook.
The eggshells in this experiment represent the enamel (outer coating) on your teeth. Toothpaste cleans your teeth and prevents stains: it removes food and drink particles that are stuck on your teeth. Teeth can be stained easily by dark-colored liquids like cola, coffee or tea. The egg without toothpaste will be brown and discolored. The egg covered in toothpaste was protected from turning brown.
Toothpaste also protects your pearly whites from decay (breaking down). The egg without toothpaste left in the lemon juice was worn down and soft to the touch, while the egg that was protected with toothpaste is stronger. The lemon juice is acidic, and those acids broke down the shell just as acidic drinks can wear away your tooth enamel. When a tooth is worn down, a cavity can form more easily. But the fluoride in toothpaste mixes with your saliva to create a protective coating around your tooth enamel. It helps keep your teeth strong and cavity-free.
Grow an Avocado Tree

For an easy lesson in Earth Science, your family can grow an avocado tree from a pit. You can buy an AvoSeedo kit , or just peel the seed and suspend it over water with toothpicks.
Get the tutorial »
Milk Bottle Xylophone

No for an experiment in sound!
- Arrange six glass jars or bottles, all the same size with no lids, in a line. What will each jar sound like when you tap it with a spoon? Make a prediction, then tap each jar. Record your observations.
- Next, put water in each of the jars. Pour 1⁄4 cup (60 ml) of water into the first jar. Add 1⁄2 cup (120 ml) of water to the second jar. Continue in 1⁄4-cup increments, adding 3⁄4 cup (180 ml) of water to the third jar, 1 cup (240 ml) of water to the fourth jar, 11⁄4 cups (300 ml) of water to the fifth jar, and 11⁄2 cups (360 ml) to the sixth jar. Add a couple of drops of food coloring to each jar.
- What will each jar sound like? Will they sound the same or different than when the container was empty? Will they sound the same or different from one another? Record your predictions.
- Tap each jar with a metal spoon. Write down your observations about each jar’s pitch (how high or low a sound is) in your notebook.
Sound waves are created by vibrations, which are back-and-forth movements that are repeated again and again. Pitch depends on the frequency of the waves — how many are created each second. A high pitch is created by high-frequency sound waves, and can sound squeaky. A low pitch is created by low-frequency sound waves, and sounds deep and booming.
When you tapped the jar, it vibrated. The vibrations traveled from the jar to the water to the air and eventually to your ears. The jars with more water had a low pitch. The sound waves vibrated more slowly because they had more water to travel through. The jars with less water had higher pitches. The sound waves vibrated faster because they had less water to travel through. A jar with no water in it makes the highest pitch because it has the least substance to travel through.
"Elephant Toothpaste"

Okay, elephants don't really brush with this stuff, which is made from a chemical reaction between hydrogen peroxide, yeast, dish soap and a few other simple ingredients. But this experiment has a big "wow" factor since, when the substances are mixed, the "toothpaste" foams out of the bottle. You can use it to teach kids about catalysts and exothermic reactions.
Get the tutorial at Babble Dabble Do »
DIY Compass

Explore the way magnetism works, and how it affects everyday objects, by magnetizing a needle and making a DIY compass. You can even spin the compass in the water, and it'll end up pointing the right way again.
Get the tutorial at STEAM Powered Family »
Craft Stick Chain Reaction

Kids can learn about the differences between potential and kinetic energy with this chain reaction. It makes a big impact: Once the tension is released, the pom poms go flying through the air!
Get the the tutorial at Science Sparks »
Color-Changing Invisible Ink

Kids will feel like super-spies when they use this heatless method to reveal pictures or colors written with "invisible ink." You can try different acid/base combinations to see which one makes the most dramatic result.
Get the tutorial at Research Parent »
Paper Bridge

Get the engineering back into STEM with this activity, which challenges kids to create a paper bridge that's strong enough to hold as many pennies as possible. How can they manipulate the paper to make it sturdier? (Hint: Fold it!)
See the paper bridge tutorial at KidsActivities.com »

Challenge your little scientist to lift up an ice cube with just a piece of string. It's possible ... with a little salt to help. Salt melts the ice and lowers the freezing point of the ice cube, which absorbs the heat from the water around it, making the water cold enough to re-freeze around the string.
Get the tutorial at Playdough to Plato »
Marshmallow Catapult

Another lesson in potential and kinetic energy, kids will love sending mini marshmallows flying in the name of science. Change some of the variables and see how that affects the marshmallow's trajectory.
Get the tutorial at Hello, Wonderful »
Leaf Breathing

It's hard for kids to picture how plants and trees "breathe" through their leaves — until they see the bubbles appear on a leaf that's submerged in water. You can also teach them about photosynthesis by putting different leaves in different spots with varying levels of sunlight.
Get the tutorial at KC EDventures »
Hoop-and-Straw Airplane
We all remember how to fold those classic, triangular paper airplanes, but these hoop-and-straw airplanes fly way better (and straighter). Experiment by changing the length of the straw and the size of the hoops and see how it affects the flight.
Get the tutorial at Mombrite »
Film Canister Rocket

Blast off! You don't need jet fuel to make these rockets go, just Alka-Seltzer tablets and baking soda, but they'll be amazed when they achieve lift-off! (Note: If you can't find old film canisters, tubes of Airborne work, too.)
Get the tutorial at Raising Lifelong Learners »
Coin Inertia

Stack up about five or so coins on a piece of cardboard and place it over a glass of water. Then, flick the cardboard out from on top of the glass. Do the coins drop into the water, or ride with the cardboard? Due to inertia, they drop into the water — a very visual (and fun!) demonstration of Newton's First Law of Motion.
Get the tutorial at Engineering Emily »
Apple Oxidation

What works best for keeping an apple from turning brown? Test to find out! Slice up an apple, and let each slice soak in a different liquid. Then take them out, lay them on a tray, and check the brownness after three minutes, six minutes and so on. Not only does this test the properties of different liquids, it also helps students practice the scientific method if they create hypotheses about which liquids would be most effective.
Get the tutorial at Jennifer Findley »
RELATED: 50 Fun Activities for Kids Will Keep Them Entertained for Hours
Coffee Ground Fossils

By making a salt dough with coffee grounds and pressing various shapes into it (toy dinosaur feet, seashells), kids can get a better understanding of how fossils are made. If you poke a hole in the top before it dries, the kids can hang their "fossils" up in their rooms.
Get the tutorial at Crafts by Amanda »
Chromatography Flowers

Chromatography is the process of separating a solution into different parts — like the pigments in the ink used in markers. If you draw stripes around a coffee filter, then fold it up and dip the tip in water, the water will travel up the filter and separate the marker ink into its different pigments (in cool patterns that you can display as a craft project). This family made the end-result even brighter by adding an LED circuit to the center.
Get the tutorial at Steam Powered Family »
Water Walking

You'll need six containers of water for this one: three with clear water, one with red food coloring, one with blue coloring, and one with yellow coloring. Arrange them in a circle, alternating colored and clear containers, and make bridges between the containers with folded paper towels. Your kids will be amazed to see the colored water "walk" over the bridges and into the clear containers, mixing colors, and giving them a first-hand look at the magic of capillarity.
Get the tutorial at Fun Learning for Kids »
Sunscreen Test

This experiment puts the A (art) in STEAM: Paint different designs on construction paper with different sunscreens, leave the papers out in the sun and compare the results. Then, hang your "conclusions" on your fridge.
Get the tutorial at Tonya Staab »
Marisa (she/her) has covered all things parenting, from the postpartum period through the empty nest, for Good Housekeeping since 2018; she previously wrote about parents and families at Parents and Working Mother . She lives with her husband and daughter in Brooklyn, where she can be found dominating the audio round at her local bar trivia night or tweeting about movies.

Parenting Tips & Advice

How to Throw a Birthday Party for a Picky Teen

150 Fun Questions to Ask Kids at Dinnertime

7 Things NOT to Do As a New Grandparent

Teacher Says Never Bring Cupcakes To School

How My Son With Disabilities Is Making Friends

What to Do When a Kid Stares at Someone

The 1,000 Most Popular Baby Boy Names Right Now

80 Best Grandma Names That Are as Unique as She Is

Why Non-Nuclear Families Are on the Rise

100 Best Disney Baby Names

140 Chic French Baby Names

The 50 Best Animated Films to Watch With Your Kids
- Children's Books
- Science, Nature & How It Works

Sorry, there was a problem.

Download the free Kindle app and start reading Kindle books instantly on your smartphone, tablet, or computer - no Kindle device required .
Read instantly on your browser with Kindle for Web.
Using your mobile phone camera - scan the code below and download the Kindle app.

Image Unavailable

- To view this video download Flash Player

Follow the author

Smithsonian 10-Minute Science Experiments: 50+ quick, easy and awesome projects for kids (Steve Spangler Science Experiments for Kids) Paperback – October 15, 2019
AS SEEN ON THE ELLEN DEGENERES SHOW ! · 50 fantastic experiments from bestselling author Steve Spangler · All experiments vetted by the scientists at the Smithsonian Institution · Step-by-step instructions with full color photos Smithsonian 10-Minute Science Experiments gives curious young readers dozens of colorful, exciting projects designed to teach them about the basics of science, physics, chemistry and engineering. They’ll learn about critical thinking, how to conduct an experiment, and how to measure results, all while enjoying themselves in a screen-free setting. Each experiment uses easy-to-find materials, most of which readers probably already have in their home. Set up and clean up is easy, and most experiments can be done in ten minutes or less! Sidebars for each experiment feature additional insights, facts and commentary. It's the perfect resource for turning curious kids into budding young scientists!
- Print length 176 pages
- Language English
- Grade level Preschool and up
- Dimensions 8.05 x 0.5 x 9 inches
- Publisher Media Lab Books
- Publication date October 15, 2019
- ISBN-10 1948174111
- ISBN-13 978-1948174114
- See all details
What other items do customers buy after viewing this item?

From the Publisher

Smithsonian 10 Minute Science Experiments
| Steve Spangler is dedicated to finding the most creative ways to make science fun and interesting for kids. With more than 1,300 television appearances to his credit, Steve is a regular guest on The Ellen DeGeneres Show, where she dubbed him “America’s Science Teacher.” | Though most projects typically take no more than 10 minutes to conduct, the book includes a number of experiments that extend the learning over an entire weekend. Each experiment includes step-by-steps instructions and photos designed to maximize comprehension and enjoyment. | Experiments range from the simple, yet surprising (Q: How many drops of water can fit on a penny? A: More than you’d imagine!) to the more complex and mind-blowing (Extract DNA from a strawberry – or build a working movie projector with a box, a magnifying glass and a mobile phone!). | Readers will learn critical thinking skills as well as how to use the scientific method to understand even more about what’s happening during each activity. Intriguing sidebars and trivia tidbits keep kids learning even when they’re not working in the “lab.” |
A Super-Cool Experiment: How to Freeze Water Instantly
| Keep the bottles close to the center of the bowl and buried in the ice as much as possible. | Scatter a generous amount of rock salt all over the surface of the ice. | Insert a thermometer into the ice between the bottles. The temperature will fall slowly over the next half hour or so. Add more ice and salt to keep the bottles buried. Once the temperature reaches 17 degrees F, move on to the next step. | After the water has been this cold for 10 minutes, gently remove a bottle from the ice. Strike the bottom of the bottle sharply against the table and ice crystals should immediately form near the top of the bottle and quickly move down through the liquid, turning it into a solid mass of ice. Twist open the cap of the second bottle and the water will freeze from the bottom up. |
Editorial Reviews
From the author, about the author.
Steve Spangler is an authority on STEM education with more than 1,800 television appearances to his credit. He’s a regular guest on The Ellen Show and the host of the three-time Emmy-nominated television series DIY Sci airing on FOX affiliates nationwide. He is a best-selling author, an Emmy award-winning television personality and a Hall of Fame Speaker Inductee, as well as the bestselling author of two previous science books for kids, Naked Eggs and Flying Potatoes and Fire Bubbles and Exploding Toothpaste . Established in 1846, the Smithsonian Institution ―the world’s largest museum, education, and research complex―includes 19 museums and galleries and the National Zoological Park. The total number of artifacts, works of art, and specimens in the Smithsonian’s collections is estimated at 156 million, the bulk of which is contained in the National Museum of Natural History, which holds more than 145 million specimens and objects. The Smithsonian is a renowned research center, dedicated to public education, national service, and scholarship in the arts, sciences, and history. www.si.edu.
Product details
- Publisher : Media Lab Books (October 15, 2019)
- Language : English
- Paperback : 176 pages
- ISBN-10 : 1948174111
- ISBN-13 : 978-1948174114
- Reading age : 5 - 10 years, from customers
- Grade level : Preschool and up
- Item Weight : 1.23 pounds
- Dimensions : 8.05 x 0.5 x 9 inches
- #44 in Children's Science Experiment Books
- #117 in Children's Mystery & Wonders Books (Books)
- #2,674 in Children's Activity Books (Books)
About the author
Steve spangler.
Steve Spangler is a bestselling author, STEM educator and Emmy award-winning television personality with more than 1,600 television appearances to his credit. Steve is also a regular guest on the Ellen DeGeneres Show where she dubbed him America’s Science Teacher.
FOLLOW Steve on all social @stevespangler.
Spangler hosts his own nationally syndicated television series called DIY Sci where viewers learn how to use do-it-yourself experiments to amaze friends. DIY Sci received 5 Daytime Emmy nominations including best educational series and best host. Spangler was inducted into the National Speaker Hall of Fame in 2010 and he holds a Guinness World Record for conducting the world’s largest science experiment in 2009.
Contact Steve by visiting https://www.SteveSpangler.com or call (855) 228-8780
Customer reviews
- 5 star 4 star 3 star 2 star 1 star 5 star 79% 14% 5% 1% 1% 79%
- 5 star 4 star 3 star 2 star 1 star 4 star 79% 14% 5% 1% 1% 14%
- 5 star 4 star 3 star 2 star 1 star 3 star 79% 14% 5% 1% 1% 5%
- 5 star 4 star 3 star 2 star 1 star 2 star 79% 14% 5% 1% 1% 1%
- 5 star 4 star 3 star 2 star 1 star 1 star 79% 14% 5% 1% 1% 1%
Customer Reviews, including Product Star Ratings help customers to learn more about the product and decide whether it is the right product for them.
To calculate the overall star rating and percentage breakdown by star, we don’t use a simple average. Instead, our system considers things like how recent a review is and if the reviewer bought the item on Amazon. It also analyzed reviews to verify trustworthiness.
Customers say
Customers find the book has many fun experiments to do. They say the directions are easy to understand and provide safety guidelines if needed. Readers also mention the book is great for kids of all ages and contains nice pictures.
AI-generated from the text of customer reviews
Customers find the activities in the book fun and easy. They say it's a fantastic way to stimulate thinking and pack learning points. Readers also mention the lava lamp experiment looks fun.
"Book contains nice pictures with great explanations for various projects . Most require things things you would find around the house...." Read more
"...There are a ton of fun experiments , and most of the supplies and ingredients are things that are around the house...." Read more
"...The other is a 8 yr boy and he loves this book and the fun experiments it has to do ." Read more
"I love that there are photos, clear instructions, and short explanations of how they work in language that most people can understand...." Read more
Customers find the directions in the book easy to understand. They also mention the projects are fun and easy to assemble.
"...The directions are easy to understand , plus they provide safety guidelines if needed." Read more
"My granddaughters (10, 9, and 7 years old) love this. They can follow the directions . Most of the items I have on hand or can easily obtain." Read more
"I love that there are photos, clear instructions , and short explanations of how they work in language that most people can understand...." Read more
"...I have most of the ingredients in my house Very easy ." Read more
Customers find the book fantastic, a great find, and worth buying.
" Awesome book with a lot of every day items...." Read more
"...It is worth buying !" Read more
"...Fun and educational activities to do on a rainy day! Great find ." Read more
" Great book . easy to understand, great pictures and information. Buy this you will not regret it!" Read more
Customers find the book great for kids of all ages.
"...I highly recommend! Fun for all ages !" Read more
" Great book for kids . Lots of fun things to do as a family." Read more
"Book arrived on time, in good shape and is great for kids of all ages " Read more
" Great book to help grandchildren to do science projects." Read more
Customers find the pictures in the book nice. They also appreciate the great explanations for various projects.
"Book contains nice pictures with great explanations for various projects. Most require things things you would find around the house...." Read more
"I love that there are photos , clear instructions, and short explanations of how they work in language that most people can understand...." Read more
"Great book. easy to understand, great pictures and information . Buy this you will not regret it!" Read more
"Easy fun science. Easy cheap ingredients and mostly picture book . Loved and used alot...." Read more
Customers find the book a great classroom gift and Christmas present.
" Classroom gift ." Read more
"Very excited to try some of the experiments in the book great gift for children " Read more
"A great hit as a Chirstmas present !!..." Read more
Reviews with images

Steve Spangler's latest book, 10 Minute Science Experiments, is his best yet!

- Sort reviews by Top reviews Most recent Top reviews
Top reviews from the United States
There was a problem filtering reviews right now. please try again later..
Top reviews from other countries
- About Amazon
- Investor Relations
- Amazon Devices
- Amazon Science
- Sell products on Amazon
- Sell on Amazon Business
- Sell apps on Amazon
- Become an Affiliate
- Advertise Your Products
- Self-Publish with Us
- Host an Amazon Hub
- › See More Make Money with Us
- Amazon Business Card
- Shop with Points
- Reload Your Balance
- Amazon Currency Converter
- Amazon and COVID-19
- Your Account
- Your Orders
- Shipping Rates & Policies
- Returns & Replacements
- Manage Your Content and Devices
- Conditions of Use
- Privacy Notice
- Consumer Health Data Privacy Disclosure
- Your Ads Privacy Choices
Science Fun

Science Experiments for Kids:
Science experiments you can do at home! Explore an ever growing list of hundreds of fun and easy science experiments. Have fun trying these experiments at home or use them for science fair project ideas. Explore experiments by category, newest experiments, most popular experiments, easy at home experiments, or simply scroll down this page for tons of awesome experiment ideas!

Making A Volcano:
Acids and Bases Can Erupt in Your Faces

Orange Fizz:
Awesome Experiments:

New Experiments:
Check Out Our Newest Experiments

Top Experiments:

Easy Experiments:

Storm In A Glass:

Home Made Play Dough:

Snow Fluff:

Snow Globe:
Squishy Turkeys:

Rainbow in a Glass:

Sizzlin’ Snowballs:

Jello Lenses:

Ice Fishing:

Super Cool Soda:

Jack-O-Cano:

Dancing Hearts:

Marbled Gift Wrap:

Massive Expanding Soap:

Surface Tension Art:

Fizzy Fruit:
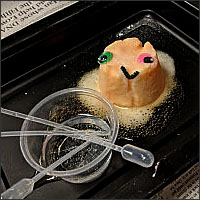
Rotting Pumpkin:

Explode A Bag:

Invisible Extinguisher:

Paper Hovercrafts:

Fun Fossil Stamps:

Cool Crystals:
Balloon Pop! Not!

Solar Eclipse Kit:

Moldy Apples:

Cool Off Volcanoes:

Vinegar Pops:

Make It Rain:

Black Light Blue Beverage:

Changing of the Leaves:

Snowflakes:
Water Fireworks:

Mind of a Student:

Balloon Speakers:

Polar Bear Blubber:
Gorgeous Gooey Gobstoppers:

Olympic Medals:

Dyed Flowers:

Rain, Rain, Don’t Go Away Gauge:

Blossoming Beans:
Butter Fingers:

Polishing Pennies:

Dancing Liquid:

Floating Egg:
Bendy Bones:

Pot Of Gold:

Layers of Liquids:

Crystal Candy:

Understanding the enhanced catalytic activity of high entropy alloys: from theory to experiment

First published on 25th June 2021
High-entropy alloys (HEAs) have evolved to be one of the most popular materials in the last decade. Their unique configuration and attractive properties make HEAs one of the most promising catalysts. Although a very limited amount of work has been reported, higher activities of HEAs than traditional catalysts have been confirmed. This review firstly summarizes the current synthetic methods of nanostructured HEA catalysts. Then, four core effects of HEAs, namely high entropy, cocktail effect, lattice distortion and sluggish diffusion, are briefly introduced, and their impacts on the catalytic properties of HEAs are highlighted. The research progress in the application of HEAs in heterogeneous catalysis is subsequently reviewed from the perspectives of both theory and experiment. The relationships among metastable microstructures, substitution effects ( i.e. strain, ligand and ensemble effects) and d-band center are discussed in detail, and their impacts on the adsorption energy of intermediates in catalytic reactions are emphasized. We conclude the review with the discussion of the challenges and opportunities of HEA catalysts. Several directions of future HEA research are put forward. The review provides a valuable resource for those interested in these exciting catalytic materials.
| Bing Wang |
1. Introduction
A promising area of research concerns the role of HEAs as heterogeneous catalysts in chemical and electrochemical reactions. The catalytic reactions are expected to be controlled by tuning the composition, surface atomic coordination and electronic configuration of HEAs. These microstructures can directly affect the interaction between intermediate species and catalysts, thus determining the catalytic activity and selectivity. Therefore, engineering the microstructures is a feasible and reasonable approach to make HEAs a promising catalyst with desirable functions. Although the field of HEAs began in 2004, 3,16 only in recent years has attention been paid to the catalytic application of HEAs. 17 Minimizing the HEA particle size to the micron or even nanometer scale is the key and challenge to the development of efficient HEA catalysts. In 2018, a thermal shock method was reported to alloy dissimilar elements into HEA nanoparticles (NPs), which greatly stimulated the blossom of HEAs in catalysis. A wide range of multicomponent nanoscale HEAs with the desired composition and size can be achieved by controlling the carbothermal shock parameters. 18
Based on the results of the limited investigations performed so far, HEAs are expected to be emerging catalytic materials in the future. In this review, the advantages and potential of HEAs as catalysts are showcased based on the current theoretical calculation and experimental results. First, we summarize the current preparation methods of nanostructured HEA catalysts in Chapter 2. The drawbacks of the current synthesis methods are highlighted. Next, the core effects of HEAs ( i.e. high entropy, cocktail effect, lattice distortion and sluggish diffusion), which play crucial roles in improving the catalytic performance, are emphasized in Chapter 3. Some unique properties of HEAs resulting from the core effects are then introduced in Chapter 4, including metastable microstructures, substitution effects ( i.e. strain, ligand and ensemble effects), d band center and adsorption energy. How these effects modify the d-band is emphasized. In particular, we discuss the mechanisms behind the remarkable catalytic activity and stability induced by these properties. Challenges associated with HEA catalysts are finally clarified, and several perspectives for future research directions and development of HEAs in the field of catalysis are suggested in Chapter 5. This work aims to be a comprehensive and critical review of general interest to communities who focus on efficient catalysts. It can provide guidance for synthesizing high-quality HEA nanoparticles with improved catalytic performance.
2. Synthesis strategies of nanostructured HEAs
2.1 carbothermal method.
| Carbothermal shock synthesis of HEA NPs on carbon nanofiber (CNF). (a) SEM of metal precursors on CNF, and the synthesized PtNi NPs after the carbothermal shock method. (b) Sample preparation and the temporal evolution of temperature during carbothermal shock. (c) Low-magnification and single-particle elemental maps, an HAADF image, and corresponding atomic maps for a PtNi alloy. (d) Elemental maps of an HEA NP composed of Pt, Pd, Ni, Co, Fe, Au, Cu, and Sn. Scale bar, 10 nm. Reproduced from ref. with permission from The American Association for the Advancement of Science. | ||
Although the carbothermal shock method can craft HEA NPs with controllable composition and ultrafine size, it only produces NPs immobilized on a carbon substrate due to the extreme synthetic conditions. It is incompatible with thermally sensitive substrates such as metal, glass, and polymers.
2.2 Electrosynthesis method
Despite many advantages, this method can only produce HEA NPs in the amorphous phase. In addition, it is difficult to achieve uniform immobilization of HEA NPs on granular supports.
2.3 Solvothermal-pyrolysis method
| (a) Schematic of the synthesis of HEA composites. (b and c) FESEM images, (d) XRD of quinary MOFs/CC, (e and f) FESEM images, and (g) XRD spectrum of HEA@ N-doped porous carbon on the surface of CC treated at 450 °C. Reproduced from ref. with permission from the Royal Society of Chemistry. | ||
Lu et al. developed a fast-moving bed pyrolysis (FEBP) strategy to synthesize HEA NPs on granular supports including carbon, γ-Al 2 O 3 and zeolite ( Fig. 3 ). This method was conducted at 923 K with a population speed of 20 cm s −1 , resulting in the formation of a small size (∼2 nm) of HEA NPs within 5 s. The critical size of HEA nucleus is small enough to reduce excess free energy used for forming nanoalloys, thus avoiding phase separation. 23 It is demonstrated that when the time for the precursors to reach the heating zone increases from 5 s to 20 s, obvious phase separation has been found in NPs. 24 In the fast moving bed pyrolysis strategy, all metal precursors with different reduction potentials can be decomposed at 923 K. 24 The formation of HEA NPs without phase separation is thermodynamically favored because of the low critical free energy. 23 In contrast, a slow moving bed pyrolysis causes a larger radius of nuclei, and thus results in phase separation. 25
| (a) Schematic diagram of the FMBP setup for the synthesis of HEA NPs. (b) Schematic diagrams for the synthesis of homogeneous and phase-separated HEA NPs by FMBP and fast bed pyrolysis (FBP) strategies, respectively. (c) The simulation of the time required for precursors/GO to reach 923 K in the FMBP process. The images in the center show the metal precursors/GO in the quartz boat. (d) HAADF-STEM images for the denary (MnCoNiCuRhPdSnIrPtAu) HEA-NPs highly dispersed on GO synthesized by the FMBP strategy; scale bar, 10 nm. (e) The HRSTEM image for the denary (MnCoNiCuRhPdSnIrPtAu) HEA-NPs (inset: Fourier transform analysis for the denary (MnCoNiCuRhPdSnIrPtAu) HEA-NPs); scale bar, 0.5 nm. (f) Elemental maps for the denary (MnCoNiCuRhPdSnIrPtAu) HEA-NPs. Scale bar, 10 nm. Reproduced from ref. with permission from Springer Nature. | ||
Iversen et al. reported a solvothermal autoclave synthesis method to synthesize HEA NPs at 200 °C in an acetone–ethanol solution with metal precursors. 26 Metal chloride salts are prone to yielding the HCP phase, whereas the acetylacetonate precursors craft the FCC phase, due to their different pre-nucleation structures. 27 Homogeneous HEA NPs were generated at a temperature lower than the reduction temperature of the metals in HEAs due to autocatalyzed metal reduction at the (111) facets of the FCC phase. 28 A Pd core is initially formed which would autocatalyze the reduction of the other metals on the (111) facets. Growth along the [111] direction occurs in the initial 20 min, whereas the growth rate along the perpendicular direction is small, inducing elongation of the HEA NPs. 28 The alloying of HEAs by solvothermal reactions, governed by the reduction rates, is a kinetically driven process rather than thermodynamic energy gain of mixing. 28 However, products of solvothermal reactions are inevitably inhomogeneous comprising particles with agglomerated crystallites deviating from the main HEA phase.
2.4 Mechanical milling approach
| Histogram distribution of Fe, Cu, Ni, Co, Cr elements in HEA NPs synthesized by the mechanical milling technique. Reproduced from ref. with permission from Springer Nature. | ||
2.5 Wet chemical synthesis
| (a) Schematic illustration of the synthesis of PtAuPdRhRu supported on XC-72 carbon and its application in HERs. (b) STEM image of PtAuPdRhRu/XC-72 carbon. (c) XRD patterns of PtAuPdRhRu/carbon synthesized by an ultrasonication-assisted wet chemistry method under different conditions. Reproduced from ref. with permission from John Wiley and Sons. | ||
Notably, the synthesis of HEA NPs by the chemical reduction strategy is challenging, particularly, when the redox potentials of individual components differ substantially. It is prone to yielding alloy NPs with severe phase separation.
2.6 Liquid metal dealloying
It has been proven that nano-porous HEAs prepared by the dealloying technique (see Fig. 6 ) are stable under both annealing and electrochemical cycling conditions due to a thin conformal oxide coating. 38,39 Moreover, such a coating improves the catalytic activity of CO oxidation by increasing the efficiency of O 2 dissociation which is the rate-limiting step for CO oxidation. Nevertheless, the liquid metal dealloying process for the preparation of the precursor alloy is a high-cost technique due to the requirement of high temperature and an inert atmosphere.
| (a and b) TEM images of the dealloyed senary AlNiCuPtPdAu at different magnifications. (c) Dark-field STEM image, (d) the corresponding SAED image and (e) STEM-EDS mapping of AlNiCuPtPdAu. The inset of (b) shows the lattice fringe of the ligaments and the formed thin oxide layer. Reproduced from ref. with permission from the Royal Society of Chemistry. | ||
2.7 Reactive sputter deposition
A major drawback of reactive sputtering is its complexity. Some fundamental aspects of the process have not been elucidated yet, which makes it difficult to understand the properties of the obtained HEA NPs as a function of the deposition conditions.
2.8 Pulsed laser ablation
Based on the above discussion, we summarize each synthetic strategy in Table 1 , including the driving force, experimental conditions, elements contained in HEAs, crystal phase and morphology, substrate applicability, duration time and elemental distribution. Most of the current methods require rigorous conditions including low pressure, temperature and inert atmospheric protection. Under such experimental conditions, HEA NPs are only immobilized on limited thermally resistant substrates ( e.g. carbon, γ-Al 2 O 3 , zeolite) rather than thermally sensitive ones ( e.g. metal foam, glass, polymer, which are often used in many applications). While some synthesis methods, such as electrosynthesis and pulsed laser ablation, yield HEAs under mild conditions, they either require targets with the same composition as the HEA NPs, or only craft amorphous NPs, narrowing the varieties of HEAs. On the other hand, it is clearly found that HEAs with good elemental distribution are generally synthesized in a short period of time, even microseconds. It is understandable that the high mixing entropy property of HEAs contributes to solid solution formation at high temperatures due to T Δ S mix . In the slow cooling process, segregation or precipitation of the second phase may occur due to decreased T Δ S mix , thus forming heterogeneous microstructures. A faster cooling rate can suppress the formation of secondary phases, and tend to create single-phase HEAs with good elemental distribution.
| Strategy | Driving force | Experimental conditions | Elements | Structure | Substrate applicability | Duration time | Elemental distribution | Ref. |
|---|---|---|---|---|---|---|---|---|
| Carbothermal shock | Electrically triggered Joule heating | 2000 K, argon | Pt, Pd, Co, Ni, Fe, Au, Cu, Sn | FCC, NPs | Carbon substrates | 55 ms | Good | |
| Electrosynthesis | Electro-shock | RT, air | Pt, La, Co, Cr, Cu, Gd, In, Mn, Ni, V | Amorphous, NPs | Graphite | 100 ms | Good | |
| Moving bed pyrolysis | Pyrolysis | 923 K, argon | Pt, Au, Ir, Sn, Pd, Rh, Cu, Ni, Co, Mn | FCC, NPs | Carbon, Al O , zeolite | 5 s | Good | |
| Solvothermal pyrolysis | Pyrolysis | 700 K, argon | Mn, Fe, Co, Ni, Cu | FCC, NPs | Carbon cloth | >5 h | Poor | |
| Mechanical milling | Mechanical force | RT, air | Ni, Cr, Co, Cu, Fe | FCC, NPs | Graphene | >80 h | Poor | |
| Wet chemistry | Ultrasonication & reduction | 973 K, nitrogen | Pt, Au, Pd, Rh, Ru | FCC, NPs | Carbon | >5 h | Poor | |
| Liquid metal dealloying | Heat & chemical reaction | >873 K, argon | Al, Ni, Cu, Pt, Pd, Au, Co, Fe, Mo | FCC, nanoporous | W/O | >5 h | Poor | |
| Reaction sputter deposition | Magnetron sputtering | 5 × 10 torr | Pt, Fe, Co, Ni, Cu, Ag | FCC, NPs | Gas diffusion electrodes | >25 h | Poor | |
| Pulsed laser ablation | Pulsed laser | RT, air | Co, Cr, Fe, Ni, Mn | FCC, NPs | W/O | ∼ms | Good |
3. Core effects of HEAs
3.1 high entropy effect.
| (a) ΔS as a function of the number of principal components for equimolar alloys. (b) Alloy definitions based on configurational entropy. (c) Structural illustration for the FCC, BCC and HCP phases of HEAs. Reproduced from ref. with permission from Frontiers. | ||
The characteristic of high mixing entropy enhances the mutual solubility among elements and facilitates the formation of simple FCC, BCC or HCP solid solution phases within HEAs during solidification ( Fig. 7c ). 50 Cantor et al. manufactured transition-metal-rich HEAs with six to nine components (the same five elements of Fe, Co, Ni, Cr, and Mn together with other elements such as Cu, Ti, Nb, Ni, Mo, Ta and Ge) in equal atomic ratio, which also forms a single FCC solid solution. 52
In principle, HEAs with a solid solution phase have many merits that can justify them as great potential catalysts. Firstly, they can be produced with wide composition ranges not available in the crystalline form, permitting the fine tuning of their electronic properties to meet catalytic reaction demands. 53 Under the definition of HEAs which consist of more than five elements, we can obtain a total of 7099 possibilities for designing equal-mole HEA systems at an arbitrary choice of a group of 13 metallic elements. 49 Unequal-mole HEAs may also be designed with minor alloying elements like AlCo 0.5 CrCuFe 1.5 Ni 1.2 B 0.1 C 0.15 for further modification of the microstructure and electronic properties. As a result, HEAs offer researchers even more room for design in terms of their composition and electronic properties than traditional alloys. Secondly, the isotropic and homogeneous characters of the alloys allow the active sites in a chemically identical environment. For HEAs, their single-phase character and lack of surface segregation of the alloying elements ensure that the catalytically active species are dispersed uniformly, which would benefit the development of the HEA catalysts with high and exclusive selectivity. 54 Thirdly, the high entropy effect has been considered to be the main reason for the stability of HEAs. The increase of the number of components significantly increases the configurational entropy, and leads to phase stability via a decrease of the Gibbs free energy. 3 It has been reported that the HCP phase of HEA catalysts (IrOsReRhRu) is still retained after heat treatment up to 1500 K and compression to 45 GPa, achieving a record temperature and pressure stability for a single-phase HEA. 55 Due to the large difference in compressibility between Os and other metals, the HEA has higher thermal expansion and lower bulk modulus in comparison with the pure metals in the compositions. 55 In the electrocatalytic oxidation of methanol, the simple-phase character and the high mixing entropy of the elements in HEAs ensure that the active sites are in a uniform dispersion in a homogeneously chemical environment, thus showing pronounced electrocatalytic activity. 55 In the nanostructures of HEA-CoMoFeNiCu, the five principal components are initially randomly assigned to each lattice site, forming a simple FCC phase. Such a solute–solution mixing phase prevents a large miscibility gap which presents in a bimetallic Co–Mo alloy. 56 As shown in Fig. 8 , the randomly mixing surface with uniform distribution of Co and Mo sites optimizes both the dehydrogenation of NH 3 molecules and the desorption of the product N 2 from the HEA surface in the catalytic decomposition reaction of NH 3 . 56
| (a) HEA catalysts preventing a large miscibility gap which presents in conventional binary alloys. (b) Schematic illustration of the rate-limiting factors in NH decomposition, labeled with dash lines in the lower panel. On a Co-rich surface (left), the rate is limited by activation or dehydrogenation of NH ; on a Mo-rich surface (right), the rate is limited by the recombinative desorption of *N; the balance for these two steps is reached on an intermediate composition with uniform distribution of Co and Mo atoms. Reproduced from ref. with permission from Springer Nature. | ||
3.2 Cocktail effect
The multi-metallic cocktail effect can optimize the electronic structures of catalysts. In HEA systems containing Ni and Pd, the electron transfer from Ni to Pd could occur due to the smaller electronegativity of Ni than Pd, which can decrease the Pd–CO binding energy and enhance the catalytic oxidation of methanol molecules. 63 Moreover, the electronic states of metal atoms are altered by alloying with the d-band center of Pd shifting down and the d-band center of Ni shifting up, promoting the electrocatalytic activity of Pd towards methanol/ethanol oxidation and enhancing the adsorption of adsorbates on the Ni sites. 64 The cocktail effect can also alter the charge transfer and chemical ordering of HEAs, as shown in Fig. 9 . In an AlCoCrCuFeNi HEA, the occupied and empty Ni 3d states shift away from the Fermi level, whereas the Cr 3d empty states shift towards the Fermi level, compared to the corresponding pure metals. 65 The charge transfer between the elements in HEAs is negligible due to the compensation of the 3d state occupancy change by the redistribution of delocalized 4s and 4p states of the transition metals. 65 These properties play important roles in the optimization of the adsorption energy of HEAs during catalytic reactions.
| The altered properties of HEAs as compared to the corresponding pure metals. A proposed scheme for the DOS redistribution of the Ni and Cr 3d bands occurring upon formation of the AlCoCrCuFeNi HEA. Reproduced from ref. with permission from Elsevier. | ||
In a word, the cocktail effect in HEAs is generally considered as a complex synergetic mechanism that is responsible for the outstanding catalytic performance of HEAs. The synergistic interactions of the multi-element compositions in HEAs have resulted in a huge divergence of the properties as compared to atoms in single-element metals. But the remarkable thing is that the mechanism of action of such multi-component synergy in HEAs remains largely unknown. The underlying synergistic mechanisms from the view point of lattice distance and electron distribution should be further explored.
3.3 Sluggish diffusion effect
The sluggish diffusion effect of HEAs can suppress the degradation caused by the coarsening of nanostructured HEA catalysts. 35,66 Despite the poor corrosion resistance of pure transition metals in strong acid or alkali solution, the high entropy alloy Ni 20 Fe 20 Mo 10 Co 35 Cr 15 composed of such elements showed high corrosion resistance in both acidic and basic electrolytes for the hydrogen evolution reaction (HER). 66 The reasons for the sluggish diffusion effect of HEAs are still controversial. Most of the researchers hold the view that multiple components are responsible for the sluggish diffusion due to low lattice-potential-energy sites provided by small atoms. 35,67 HEAs with multiple principal elements have larger fluctuations in lattice potential energy than pure metals or traditional alloys ( Fig. 10 ). Many low lattice-potential-energy sites always serve as atomic traps and blocks. 67 Thus, if an atom jumps to a low-local-potential state, it would have a low possibility to jump out. In contrast, if an atom jumps to a high-local-potential state, it would have a high chance to hop back to the initial site. Both cases can hinder atomic diffusion and particle coarsening due to the increased energy barrier and activation energy for diffusion. Meanwhile, some other researchers think that certain elements, such as Mn in CoCrFeMnNi alloy, produce a deep potential wall and thus cause a sluggish effect. 67 Therefore, a thorough study should be performed to clarify the origins of the sluggish diffusion effect of HEAs, which in turn helps researchers better design the overall architecture of high-performance HEA catalysts.
| Schematic diagram of the variation of low-potential energy and mean difference (MD) during the migration of a Ni atom in different matrices. The MD for pure metals is 0, whereas that for HEA is the largest. Reproduced from ref. with permission from Elsevier. | ||
3.4 Lattice distortion effect
| The DFT simulation results of (a) the pristine lattice with an ideal FCC structure and (b) the distorted lattice of the CoCrFeNi alloy. Reproduced from ref. with permission from Frontiers. (c) Schematic of the advantages of the lattice-distortion alloys for bifunctional oxygen electrocatalysts. Reproduced from ref. with permission from Elsevier. | ||
It was reported that the lattice distortion in an Fe-enriched alloy promoted a higher density of active electrons around the Fermi level ( Fig. 11c ). 69 The higher density of activated electrons results in faster electron transfer. In contrast, the electron transfer in alloys with a pristine lattice is quite limited due to the absence of activated electrons. Thus, the alloys with lattice distortion showed improved catalytic performance than alloys with a pristine lattice in catalysis, such as the oxygen evolution reaction (OER) and oxygen reduction reaction (ORR). 69 Moreover, some metastable structures (namely fragmented domains such as short-range order, critical defects, amorphous structures, etc. ) may form to accommodate the lattice distortion effect during the preparation of HEAs. 1 Such metastable microstructures are believed to play a key role in enhancing the catalytic performance of HEAs. Lattice distortion can also induce a residual strain field with atomic scale fluctuation, shift the d-band center of catalysts and ultimately affect the catalytic selectivity. The effects of these microstructures and strain on the catalytic performance of HEA catalysts are discussed in detail in Section 3.2 and 3.3, respectively.
In brief, the high-entropy effect simplifies the microstructures of HEAs to form simple solid solution phases, leading to the homogeneous distribution of active site configurations in catalysis. The cocktail effect causes a synthetic effect on properties, wherein the interactions among the different elements may optimize the adsorption energy of the intermediates, thereby enhancing the catalytic activity. The sluggish diffusion effect increases the activation energy and reduces the coarsening kinetics in the grain growth process. It enhances the thermal and chemical stability of HEAs in catalysis. The lattice distortion effect has a great impact on the physical and chemical properties of HEAs, which often results in severe strain due to the atomic size mismatch. Such a strain potentially shifts the d-band center of alloys, and affects the binding modes of intermediates as well as the catalytic selectivity. Moreover, some possible metastable microstructures in the solid-state solution phase of HEAs may form during the preparation process due to the lattice distortion effect, which holds promise for providing a variety of active sites in catalysis.
4. Core effect-induced properties for highly efficient HEA catalysts
| HEAs | Phase | Synthetic method | Reaction | Mechanisms for improved performance | Ref. |
|---|---|---|---|---|---|
| PtFeCoNiCuAg | FCC | Sputter | Electrocatalytic methanol oxidation | Not mentioned | |
| PtNiCoCuFe | Not mentioned | Electrosynthesis | Electrocatalytic methanol oxidation | Resistance to the poisoning of carbonaceous species | |
| IrOsReRhRu | HCP | Pyrolysis | Electrocatalytic methanol oxidation | Not mentioned | |
| NiNbPtSnRu | Amorphous | Mechanical milling | Electrocatalytic methanol and CO oxidation | Lower rate of poisoning | |
| PtRuCoOsIr | FCC + HCP | Dealloying | Electrocatalytic methanol oxidation and the ORR | The downshift of the Pt d-band weakens the O–O bond | |
| AlCoCrTiZn | BCC | Mechanical milling | Catalytic degradation of azo-dyes | Lattice distortion and residual stress lead to a low activation energy barrier | |
| AlCrFeMnTi | FCC + BCC | Mechanical milling | Catalytic degradation of azo-dyes | The presence of plenty of nano-galvanic cells among the principal elements | |
| AuAgPtPdCu | FCC | Mechanical milling | Electrocatalytic CO reduction | Destabilization of *OCH intermediates and the strong stabilization of *O intermediates | |
| NiFeMoCoCr | FCC or FCC + μ | Arc-melting | Electrocatalytic HER | High coordination numbers of single-phase FCC promote the hydrogen adsorption | |
| FeCoPdIrPt | FCC | Moving bed pyrolysis | Electrocatalytic HER | The downshift of Pt antibonding states facilitates hydrogen species desorption | |
| IrPdPtRhRu | FCC | Polyol method | Electrocatalytic HER | Deeper d-band centre locations (between Ir and Pt) | |
| MnFeCoNiCu | FCC | Solvothermal-pyrolysis | Electrocatalytic OER | The presence of lattice defects contributes to the catalytic activity | |
| AlNiCoFeX (X = Mo, Nb, Cr) | FCC | Top-down synthesis | Electrocatalytic OER | X atoms prefer high formal oxidation states, facilitating proton migration to O at Ni/Co sites | |
| CoFeLaNiPt | Amorphous | Electrosynthesis | Electrocatalytic HER and OER | Synergism between Pt and the other elemental components on the atomic scale | |
| PtAuPdRhRu | FCC | Wet chemistry | Electrocatalytic HER and OER | High-entropy at the nanoscale and strong synergistic effects between active metals | |
| AlNiCoIrMo | FCC | Dealloying | Electrocatalytic HER and OER | The increased covalency of Ir–O bonds by alloying | |
| CrMnFeCoNiNb | Not mentioned | Sputter | Electrocatalytic ORR | Solid solution phase with altered properties overcomes the limitations of the single elements | |
| AlCuNiPtMn | FCC | Dealloying | Electrocatalytic ORR | The best electronic modulation for the Pt surface through surface strain and/or ligand effects | |
| PtPdFeCoNi | FCC | Carbothermal shock | Electrocatalytic ORR | Rapid electrochemical screening is demonstrated by using a scanning droplet cell | |
| Hollow RuIrFeCoNi | FCC | Droplet-to-particle | As the cathode catalyst for Li–O batteries | Maximizing the material usage efficiency by tuning the HEA shell thickness | |
| FeCoNiCuMo | FCC | Carbothermal shock | Thermocatalytic NH decomposition | Tunable surface adsorption properties by varying the Co/Mo ratio | |
| RuRhCoNiIr | FCC | Carbothermal shock | Thermocatalytic NH decomposition | A synergistic effect from multiple elements, ultrafine size, and homogeneous structure | |
| PtPdRhRuCe | FCC | Carbothermal shock | Thermocatalytic NH oxidation | Homogeneous nature of the solid-solution NPs |
4.1 Metastable microstructures
| Phase transformation during the solidification of an HEA. | ||
An interesting issue arises regarding the functions of the metastable microstructures in catalytic reactions. Alloys with these microstructures have coordinatively unsaturated sites which are essential for the bonding and activation of the reactants. HEAs have a high concentration of coordinatively unsaturated metal centers (active sites), which makes adsorption 83 and surface reactions 84 easier than on the corresponding crystalline catalysts. Although the effect of such microstructures on the catalytic properties of HEAs has not yet been reported in the literature, their functions in traditional metal/alloy-based catalysts have been demonstrated in detail. 85–87 It has been reported that the presence of unsaturated Ni( II ) binding sites in a nano-porous hybrid material significantly improved hydrogen sorption quantity compared with that of similar materials without unsaturated metals sites. 83 A silver catalyst with stacking faults (defects) and a low coordination number showed superior activity of the hydrogen evolution reaction that outperforms commercial platinum on carbon which is usually considered as the best catalyst. 88 These sites make the adsorption and surface reactions of catalytic intermediates easier than conventional crystalline catalysts, ensuring a high catalytic activity. Catalytic doping of alloys is an effective avenue to improve the hydrogen-storage property of MgH 2 . Compared with crystalline HEAs, amorphous counterparts exhibited better kinetics and lower activation energies during MgH 2 catalysis due to a more uniform distribution of highly refined nanostructures in the amorphous phase. 77 The amorphous alloy catalysts become brittle by absorbing hydrogen, which is beneficial to the formation of refined nanostructures. Consequently, the presence of an amorphous HEA catalyst with the refined nanostructures accelerates hydrogen diffusion in the Mg/MgH 2 matrix, thus enhancing the hydrogen storage properties of MgH 2 . In addition, as the metastable structures are nonporous, the surface reaction would not be affected by diffusion limitations, which is often a problem in traditional heterogeneous catalysis. 89 All these features make HEAs with metastable microstructures attractive in heterogeneous catalysis.
4.2 Relationships of strain, ligand and ensemble effects with the d-band of HEAs
| Three substitution effects in alloys: strain, ligand and ensemble effects. | ||
The mechanical behavior of catalysts is one of the most important factors for the reliable and efficient catalytic reactions, and understanding the role of surface strain in tuning the reaction is critical for catalyst design. 92–97 For instance, as-exfoliated monolayered WS 2 nanosheets exhibited enhanced electrocatalytic activity for hydrogen evolution due to the high concentration of the strained metallic 1T phase. 98 In principle, strain modifies the physical/chemical properties of catalyst surfaces by changing the average energy of the d band. 99 The width of the surface d band was found to be proportional to the interatomic matrix element that describes bonding interactions. The misfit strain changes the width of the d band through changes in the d-orbital interactions between the d orbitals of a metal atom and the d orbitals of its nearest neighbors that are quite sensitive to interatomic spacing. 100 As a consequence of the d width change, the average energy of the d band (the d-band center) moves down or up relative to the Fermi energy in order to maintain a constant d-band filling, resulting in modifications of the strained surface properties. On the basis of the theoretical simulations, the d-band center of catalysts play an important role in their catalytic activity because the d-orbital electrons determine both bond formation and breaking of the intermediate species. 101,102 According to the d-band model, 103 the position of the d-band center determines the adsorption energies and activation energy barriers. The shift of the d-band center influences the bonding and anti-bonding states of adsorbates and reactants on the alloy catalyst surface, and further determines the activity and selectivity of catalytic reactions. 104 To achieve optimal catalytic activity, the d-band center must not be too close or too far from the Fermi level. Notably, the shift trend of the d-band center is reverse for late transition metals (LTMs, for which the d bands are more than half filled) and early transition metals (ETMs) with a less than half-filled d band. Fig. 14 shows the influence of tensile strain on the position of the d band in ETMs and LTMs. 105 ETMs exhibited lower adsorption energies under tensile strain because the expanded lattice reduced the overlap of the wavefunctions and therefore narrowed the metal d band, in contrast to what is observed in LTMs. 105,106 The band narrowing results in an increased population of the d band of LTMs, upshifting the d-band center to preserve the degree of d-band filling. Yan et al. proved that the influence of externally applied elastic strain on the catalytic activity of metal films in the hydrogen evolution reaction (HER) is in a controlled and predictable way. 107 The activities of Ni and Pt were accelerated by compression, while that of Cu was accelerated by tension. 107 Pt-based catalysts exhibited intensive adsorption for catalytic intermediates in the HER and ORR, which was weakened by introducing compressive strain through interface mismatch or size reduction. 108–111 It has been reported that a surface strain of −2.0% would be the best for Pt-based alloy catalysts toward the highest ORR activity. 112,113 DFT has proven that a Pt layer with −2.0% strain would lead to 30 times higher ORR activity than pure Pt. 114 In the experiment, the 3ML-Pt/Pt 25 Ni 75 (111) alloy with −1.7% strain (close to the best strain of −0.2% in theoretical predictions) displayed enhanced activities for the ORR, consistent with the theoretical results. 83 For the FeCoPdIrPt system in the HER, 24 Fe, Co and Pd could downshift the d-band center of Pt, and more electrons could occupy the antibonding states, facilitating the desorption of hydrogen species to produce more hydrogen than the commercial Pt/C catalyst.
| The effect of (a and c) tensile and (b and d) compression strain on the position of the d band in early transition metals and late transition metals. | ||
Considering that catalytic materials are usually nanoscale and the absolute magnitude of the induced strain is very small, strain along a certain direction is defined as ( l f − l i )/ l i , where l i and l f represent the atomic bond length in the initial and final states, respectively. 105 It has been proven that the maximum catalytic activity required an optimum atomic bond length in catalysts. 115 A larger atomic bond length would cause oxygen dissociation before the adsorption in ORRs, whereas a smaller value would generate strong repulsive forces for dual-site adsorption. 115 Furthermore, an optimized surface strain can contribute to a high HER performance due to the small hydrogen adsorption Gibbs free energy on the catalytic sites. 116 Strain-induced changes in the atomic bond length can be viewed as the bulk lattice distortions in catalysts. 105 For HEAs, a serious lattice distortion effect gives rise to changes in the surface strain. The surface strain of HEAs can be tuned by selecting principal components with different atom radii. A seven-component FeNiCoSiCrAlTi HEA coating with BCC solid solution phase was prepared by laser cladding on a low carbon steel substrate. 117 The presence of the small-atomic-radius Si and large-atomic-radius Al and Ti increased the lattice packing density and crystal distortion. The segregation of Ti atom caused the different lattice expansion and growth stress between Ti-depleted polygonal grains and Ti-enriched interdendrites, which can lead to increase of the contraction stress at their interfaces. 117 A higher concentration of Al, which has a larger atomic radius than Co, Ni, Fe, and Cu elements, resulted in a larger lattice distortion of Co 25 Ni 25 Fe 25 A l7.5 Cu 17.5 with transformation of the HEA phase from FCC into a BCC structure. 118–120 This result revealed that the distribution of random elements with various atomic radii in HEAs can induce volume contraction or expansion along the specific direction. In addition, the local lattice distortion can result in the fluctuation of stacking fault (SF) energy. 121,122 The SF energy can change the number of SFs in catalysts, and thus tune their catalytic properties. 16 A high density of SFs in silver NPs caused a low coordination number and high tensile strain, transforming the non-active Ag into a highly active catalyst towards the HER. 116 The different atomic radii of the principal elements in HEAs can induce local strain effects due to the variation of SF energy. 34 SF–SF intersections produced a local strain field, leading to dislocation accumulation and SF formation in order to release the local strain concentration. 34 Based on the above mentioned analysis, it is expected that HEAs can optimize the surface strain, surface d-band width and SF density by selecting principal components with different atomic radii to achieve high catalytic activity. 123
| (a) The ligand effect of HEAs and the d-band center shift. The green balls represent noble metal atoms. The orange, purple, blue and yellow balls represent non-noble metal atoms. (b) Changes in the d-band centers for monolayer overlayer on transition metal substrates. Reproduced from ref. with permission from Elsevier. | ||
Owing to the ligand effect, the reactivity of one metal can be varied substantially by depositing it on another due to the adjustment of the d-band. Fig. 15b displays the d-band center change of a given metal when it is deposited on another metal, calculated by DFT. 125 This helps us to design HEAs with suitable components for a specific catalytic reaction. For instance, Pt is generally used as an anode catalyst for PEM fuel cells. However, the strong binding of CO on the Pt surface leads to poisoning of the catalyst. Finding a catalyst surface that weakly binds CO is desirable. As shown in Fig. 15b , a surface with weaker CO bonds than Pt(111) can be obtained by positioning Pt on top of atoms such as Ir, Rh, Ru, Cu, Fe, and Co, due to the down-shift of the Pt d-band. 125 Likewise, if a surface with stronger adsorption energy of intermediates is required, Pt can be put on atoms such as Ag or Au to up-shift the d-band center.
As lattice strain often changes the electronic structure of alloys, the strain effect and the ligand effect are difficult to be distinguished in practical HEA catalysts. To understand the contribution of the ligand effect, Rossmeisl et al. have isolated the electronic ligand effect of the HEA catalyst (IrPdPtRhRu) from the strain effect by creating an unstrained environment in DFT to investigate its ORR activity ( Fig. 16 ). 126 After statistical analysis of 2000 DFT calculations and subsequent host/guest calculations, it has been found that selected atoms among the fourth nearest neighboring positions in the third layer of an FCC (111) metallic structure have more impact on the bond strength of an adsorbate in the ORR than any second or third nearest atomic positions. 126 It is found that the ligand effect affects both the d-band center and d-band shape which correlates closely with the bond strength of the adsorbate.
| Schematic of atomic positions grouped by layer and distance from the binding site on an FCC (111) surface microstructure for (a) on-top adsorption on Pt and (b) fcc hollow site adsorption on IrPdPt. The fourth layer contains zones 4A, 4B, 4C and 4D and has an identical layout to the first layer. (c) Overview of the regression coefficients of the least squares fits for each element by zone. The atoms with direct coordination (i.e., zones 1A and 2A) to the binding atoms have a large effect on the binding energy of the intermediate. Zone 3B has a similar sized impact on the binding energy to the neighbors in zone 2A. Reproduced from ref. with permission from John Wiley and Sons. | ||
Alloys with distinct atomic ensembles result in different absorber binding strengths. When Au alloys with Pt as the catalyst in allyl alcohol hydrogenation, the H atoms are only adsorbed onto Pt sites. 127 Au atoms, which simply act as a surface diluent, have no impact on the H binding energy. PtAu catalysts exhibit a linear increase in activity with increasing Pt ratios. 127 However, when Pt atoms are replaced by Pd, H can interact with both Pd and Au atoms. Thus, the binding energy strength of H atoms on the Pd–Au atomic surface ensemble can be tuned to achieve improved hydrogenation activity. The contrasting behavior of PtAu and PdAu alloys is because different effects work in these two systems. The ensemble effect dominates the PtAu system, whereas PdAu alloys exhibit both ensemble and ligand effects. The ligand effect results in the direct charge transfer from Au to Pd atoms, leading to the d-band perturbation. These results provide valuable guidance for tuning the absorber binding energy by screening suitable components in HEAs.
Overall, the strain, ligand, ensemble effects and the d-band perturbation can significantly affect the adsorption energy of intermediates in catalytic reactions. The following section mainly discusses the relationship between the adsorption energy and catalytic activity of HEA catalysts.
4.3 Adsorption energy
| (a) Scheme of active site distribution within one adsorption peak. (b) Visualized intrinsic current response in the kinetic region of these grouped active sites, considering their activity as well as the intensity. (c) Scheme of correlations between a complex solid solution (CSS) NP structure, its effect on the adsorption energy distribution pattern, and the respective electrochemical response in the kinetic region. Reproduced from ref. with permission from John Wiley and Sons. | ||
The optimization of adsorption energy has also been found when a nanocrystalline AuAgPtPdCu HEA was used for the electrocatalytic reduction of CO 2 . 9 The faradaic efficiency is near 100% with respect to gaseous products at −0.3 V vs. reversible hydrogen electrode (RHE) due to a large number of catalytic sites present randomly on the surface of the HEA NPs, highlighting the uniqueness of the HEAs. 9 In CO 2 reduction, the conversion of *OCH 3 into the *O intermediate, which is an endoergic reaction, has been considered the rate-determining step due to the high barrier. DFT calculation, based on the free-energy calculations of intermediates, demonstrated that the barrier is much lower for the HEA system (1.35 eV) than for the pristine Cu(111) (1.95 eV), as shown in Fig. 18 . 9 The fact that the CO 2 reduction on the HEA NPs over the Cu(111) is thermodynamically favored should be attributed to the easier destabilization of *OCH 3 intermediates and the stronger stabilization of *O intermediates on the HEA surface than the Cu(111) surface. For stabilization of *O, both Pd 11 and Cu 7 atoms can bond O atoms on the HEA NP surface. 9 Notably, despite the presence of five elements in the HEA catalyst, the electrocatalytic activity is predominantly described by Cu atoms, and other atoms only provide a synergetic effect. 9
| (a) Schematic of the electrocatalytic reduction of CO on the surface of a AuAgPtPdCu HEA. (b) X-ray diffraction (XRD), (c) transmission electron microscopy (TEM) bright-field image, and (d) high-resolution scanning transmission electron microscopy (HR-STEM) image of AuAgPtPdCu HEAs; the inset of (c) shows a high magnification image of a single AuAgPtPdCu HEA nanoparticle. (e) Chemical homogeneity of Au, Ag, Pt, Pd, and Cu. (f) Optimized structure of the special quasi-random structure of the AuAgPtPdCu HEA. (g) Free-energy diagram of CO reduction reaction on the AuAgPtPdCu surface. The inset shows the optimized structures of the intermediates on the HEA surface. Gray, green, pink, yellow, blue, brown, red, and orange spheres represent Pt, Pd, Ag, Au, Cu, C, O, and H atoms, respectively. Reproduced from ref. with permission from the American Chemical Society. | ||
Electrochemical HER is a classic two-electron-transfer reaction occurring through the Volmer–Heyrovsky mechanism. This mechanism involves two steps: Volmer step (H + + e − + * → H*) and Heyrovsky step (H + + e − + H* → 0.5H 2 + *). The corresponding free energy changes of the two steps are given by Δ G Volmer = E H ads + Δ E ZPE − T Δ S H and Δ G Heyrovsky = − E H ads − (Δ E ZPE − T Δ S H ), where E H ads is the hydrogen adsorption energy, Δ E ZPE and Δ S H are the difference in zero-point energy and the entropy difference between the adsorbed state and H 2 , respectively. 133 Thus, E H ads determines the overall HER activity. It has been found that the catalytic activity shows a volcano trend as a function of the hydrogen adsorption strength on catalyst sites ( Fig. 19a ). 134 The activity can be optimized when the adsorption energy of hydrogen species on catalysts is close to 0 V due to the balance of adsorption and desorption. 97 Although Pt is a high-activity catalyst for the HER, its scarcity limits its application. HEAs are expected to decrease the loading of noble metals without losing their electrocatalytic efficiencies as the cocktail effect can optimize the adsorption strength of hydrogen. In the FeCoPdIrPt system, the combination of Co with strong adsorption and Ir with weak adsorption can moderate the free energy of H species. 24 Pd, despite its poor HER activity, can modulate the hydrogen binding energy on the Pt surface. 97 Thus, the synergic effect of the atoms in the HEA NPs leads to excellent activity towards the HER. 24 In another work, an amorphous HEA of CoFeLaNiPt has also been found to show improved electrocatalytic performance compared to the individual components during the HER due to the elemental synergisms between Pt and other components on the atomic scale ( Fig. 19b ). 6
| (a) Current density as a function of hydrogen adsorption energy. Reproduced from ref. with permission from the American Chemical Society. (b) Electrocatalytic O and H evaluation of a CoFeLaNiPt HEMG-NP (High-Entropy Metallic Glasses-nanoparticle) electrocatalyst. Each material was loaded onto the HOPG (highly oriented pyrolytic graphite) substrate. Reproduced from ref. with permission from Springer Nature. | ||
The ORR in fuel cells usually involves four-proton–electron transfer to form H 2 O. 135 During this process, the adsorption energy of intermediates such as O, OH and OOH on the surface of catalysts determines the catalytic activity of the ORR. The activity of metals with strong adsorption of the intermediates is limited by the proton transfer to the intermediates. For metals with weak adsorption of the intermediates, oxygen is unstable on the catalyst surface, thus no transfer of protons and electrons to oxygen occurs. A theoretical volcano-like trend between the activity and intermediate adsorption energy is also observed ( Fig. 20a and b ). Although Pt is near the top of the volcano-like trend, there still exists an overpotential of ca. 0.4 V for the ORR. 136,137 Pt-based alloys can reduce the overpotential by optimizing the intermediates' adsorption energy related to the pure Pt catalyst. 138,139 A first principles study has proven that transition metals can weaken the adsorption of chemical species to the Pt, leading to higher ORR activity. 140 Fig. 20c compares the calculated free energies of the steps in the ORR on CuNiPt and CuNiPtMn HEA surface systems. 79 The generation of the OOH* intermediate is the rate-limiting step of the ORR on both catalyst surfaces. Compared with CuNiPt, the addition of Mn in CuNiPtMn modulates the electronic properties of the catalyst surface, and thus optimizes the binding of OOH through the cocktail effect, resulting in enhanced activity ( Fig. 20d and e ). 79 The cocktail effect has also been found to be applicable to design advanced ORR catalysts without Pt, such as AlCuNiAgMn, AlCuNiAgMo, and AlCuNiAgCo, which even outperform the pure Pt catalyst. 79 The fact that the cocktail effect can optimize the adsorption energy of intermediates in the ORR is also proven by density functional theory (DFT), as shown in Fig. 21 . 141 In order to achieve a highly efficient ORR reaction, *OH and *O intermediates must not be too stable on the surface of catalysts nor be easily removed. While Pt(111) is the best crystal surface of pure metal for the ORR, the adsorption energy of *OH (∼0.1 eV) on it is still too strong. 136 According to the DFT results, the surface of an HEA catalyst can offer a near-continuous distribution of adsorption energy due to the complicated surface configurations. 141 Thus, a high-efficiency ORR can be achieved by tuning the compositions of the HEA catalyst with optimal adsorption energy close to the peak of the Sabatier volcano curve. 142 The HEA materials become a design platform for new alloys by increasing sites with superior catalytic activity to pure Pt(111).
| Trends in ORR activity as a function of (a) the O adsorption energy or (b) both the O and the OH binding energy. Reproduced from ref. with permission from the American Chemical Society. (c) Free energy profiles of the ORR steps on the PtCuNiMn and PtCuNi. Adsorption configurations of the OOH on the (d) PtCuNi and (e) PtCuNiMn models. The colors dark blue, pink, brown, green, red and white represent the atoms of Pt, Mn, Cu, Ni, O and H, respectively. Reproduced from ref. with permission from Elsevier. | ||
| (a) *OH on-top binding. Orange (1): binding site. Light green (2): surface neighbors are coordinating once to the binding site. Light gray (3): subsurface neighbors are coordinating once to the binding site. (b) *O FCC hollow site binding. Dark green (4): surface neighbors are coordinating twice to the binding site. Dark gray (5): subsurface neighbors coordinating twice to the binding site. Distribution of adsorption energies for (c) Ir Pd Pt Rh Ru , (d) Ir Pd Pt Rh Ru , (e) Pd Ru , and (f) Ir Pt (global maximum activity). A represents the activity. Reproduced from ref. with permission from Elsevier. | ||
5. Conclusions and perspectives
Firstly, scientific theories for the construction of HEAs is scarce. There are a huge number of possible compositions and combinations of properties in the HEA field. Wise element design strategies for suitable compositions and structures to fit the requirements in heterogeneous catalysis thus become especially important. The rational design of HEA nanomaterials from fundamental principles has the potential to create catalysts with high activity and selectivity. 147 Until now, designing new HEA materials is based on the traditional trial-and-error method which becomes very difficult due to the large number of possible compositions for HEAs. As the combinations of composition and process for producing HEAs are numerous, each HEA has its own microstructure and properties to be identified and understood. It is very important to present basic concepts relating to HEAs in advance. Using principles of materials science is the most basic way to design a new material. This route can be used at the beginning to develop new HEA nanomaterials for desired properties by fully understanding the properties of components in materials, such as the crystal structure, atomic size, atomic weight, redox potential, electronegativity, melting and boiling points, density, electron configuration, etc. Rapid-throughput screening approaches are required to vet potential HEA compositions. For example, if we want to design low-density HEAs, more light elements should be used. If HEAs with oxidation resistance are needed, more oxidation-resistant elements such as Al, Cr, and Si should be selected. If HEAs with BCC phases are desired, Al can be added by strong binding with other elements to promote the formation of a BCC phase. In catalysis, Pd instead of Pt can be chosen as one of the components in HEAs to promote the catalytic activity due to the high electro-oxidation catalytic activity and lower price of Pd. In addition, both leverage neural network (NN) and machine learning can be employed to provide insights for rational design of HEA catalysts. The NN strategy can account for the three substitution effects of HEAs (mentioned in Fig. 13 ) for predicting the adsorption energy of catalysts. 148 Machine learning can help calculate adsorption energies of all surface sites on catalysts, allowing the optimization of the HEA compositions. 9,76 Nevertheless, there is also a risk that promising alloys might be dismissed in the early stage by these methods, due to the absence of exploration of the complex links between microstructures and properties. Certainly, careful experimental assessment of HEAs is the prerequisite to obtain the microstructure characteristics and stability.
Secondly, moderate and scalable synthesis strategies are urgently needed. Although current methods mentioned in Chapter 4 have successfully crafted HEA NPs, they generally require rigorous conditions such as high pressure, temperature and inert atmospheric protection. NPs can be only immobilized on limited thermally resistant substrates rather than thermally sensitive ones. It may incur great stoichiometric deviation due to the high vapor pressure of metal elements under these extreme conditions. The mild electrosynthesis method can only craft amorphous NPs (electrosynthesis method 6 ) or is limited by the corresponding targets (PLA approach 44 ). It is urgent to develop a more convenient technology with low energy consumption under mild conditions for synthesizing a library of HEA nanostructures.
Thirdly, although HEAs have shown great potential in catalysis applications, the understanding of the whole HEA world and how HEA materials behave in complex catalysis environments is still at the infant stage. Several future trends are pointed out: more fundamental studies on HEA structures are required . In most cases, the catalytic performance of HEA catalysts is simply explained by the synergetic effect between multiple elements. Very limited studies aim to establish an in-depth understanding of the structure–performance relationship. HEA structures with mutual interactions between different atoms, lattice distortion, metastable structures, stacking fault energy, electrical and thermal conductivity, diffusion coefficients, corrosion and oxidation are desired to fully understand the relationship between the structure and performance. Moreover, a theoretical model including microstructure details in HEAs will be instructive for establishing an accurate structure–performance relationship over HEA catalysts. Architectures in the atomic structures of HEAs can be explored in a systematic and site-specific manner. Otherwise, the possibility of comprehensively exploring such systems would be precluded. Accurate models for catalyst selections need to be built . The lack of such models makes the design of high-activity HEA catalysts difficult. The chosen descriptors determined the catalytic activities of catalysts. For example, the Sabatier principle shows a volcano-type relationship between catalytic activities and adsorption energy, whereas it is a linear relationship in the Brønsted–Evans–Polanyi behavior. 149,150 Due to the interactions of elements in alloys, the properties in terms of adsorption energy may be more complex. Establishing a descriptor suitable for HEA materials will contribute to guiding the selection of high-activity catalysts. The behavior of HEA NPs in real service environments is investigated . In the application of industrial catalysis, critical environments, such as high temperature/pressure, oxidizing/reducing conditions, and extreme pH solution, are usually involved. 151 Since it is challenging to obtain structural and compositional information of such nanoscale HEAs at spatial and temporal resolution, there is still very limited knowledge of the behavior of HEA NPs in these environments. Shahbazian-Yassar et al. employed in situ gas-cell TEM to investigate the oxidation behavior of Fe 0.28 Co 0.21 Ni 0.20 Cu 0.08 Pt 0.23 HEA NPs at 400 °C and in an atmospheric pressure air environment ( Fig. 22 ). 152 Although the overall oxidation kinetics of the HEA NPs are slower than that of both monometallic and bimetallic alloy NPs with similar principal elements, the oxidation of HEA NPs governed by the Kirkendall effect has been found involving outward diffusion of Fe, Co, Ni, and Cu to form an oxide layer with a concentration gradient. Pt stays in the HEA core region during the oxidation process due to its nonreactivity. Studies on other extreme conditions such as oxidizing/reducing conditions and extreme pH solution are also needed, yet are still scarce, but are crucial for understanding HEA behavior in these environments and providing insights in designing chemical/thermal corrosion-resistant alloys for various applications.
| (a) Schematic of the in situ gas-cell in air. (b) In situ TEM image sequences of HEA NPs during annealing in air to study the oxidation of HEA NPs. (c) Exemplary atomic model for an oxidized slab after equilibration. (d) Schematic illustration of the oxidation process of HEA NPs. Reproduced from ref. with permission from the American Chemical Society. | ||
Fourthly, more research can be focused on high-entropy ceramics (HECs) such as nitrides, carbides, oxides, and sulfides. A few studies have indicated that four core effects of HEAs are also applicable in HECs. As some conventional phosphides/sulfides/oxides/carbides/nitrides have been proven as efficient catalysts, 153–157 HECs are expected to have promising applications in catalysis via their four core effects. Unfortunately, there are few reports on the synthesis of HEC nanomaterials, limiting their research in the field of catalysis.
Based on these deliberations, HEAs have thrust us into a new world of seemingly infinite possibility. Research on HEA-based catalysis has been attracting increasing attention. Recent studies indeed offer significant potential to improve our fundamental understanding of the catalytic behavior of HEAs. Nevertheless, future efforts should focus on key features presented by the microstructures of HEAs, rather than wandering in its limitless expanse.
Conflicts of interest
Acknowledgements.
- Y. G. Yao, Z. Y. Liu, P. F. Xie, Z. N. Huang, T. Y. Li, D. Morris, Z. Finfrock, J. H. Zhou, M. L. Jiao, J. L. Gao, Y. M. Mao, J. W. Miao, P. Zhang, R. Shahbazian-Yassar, C. Wang, G. F. Wang and L. B. Hu, Sci. Adv. , 2020, 6 , eaaz0510 CrossRef CAS PubMed .
- X. Z. Wang, Q. Dong, H. Y. Qiao, Z. N. Huang, M. T. Saray, G. Zhong, Z. W. Lin, M. J. Cui, A. Brozena, M. Hong, Q. Q. Xia, J. L. Gao, G. Chen, R. Shahbazian-Yassar, D. W. Wang and L. B. Hu, Adv. Mater. , 2020, 32 , 2002853 CrossRef CAS PubMed .
- J. W. Yeh, S. K. Chen, S. J. Lin, J. Y. Gan, T. S. Chin, T. T. Shun, C. H. Tsau and S. Y. Chang, Adv. Eng. Mater. , 2004, 6 , 299–303 CrossRef CAS .
- D. B. Miracle and O. N. Senkov, Acta Mater. , 2017, 122 , 448–511 CrossRef CAS .
- Y. Zhang, Y. J. Zhou, J. P. Lin, G. L. Chen and P. K. Liaw, Adv. Eng. Mater. , 2008, 10 , 534–538 CrossRef CAS .
- M. W. Glasscott, A. D. Pendergast, S. Goines, A. R. Bishop, A. T. Hoang, C. Renault and J. E. Dick, Nat. Commun. , 2019, 10 , 2650 CrossRef PubMed .
- P. F. Xie, Y. G. Yao, Z. N. Huang, Z. Y. Liu, J. L. Zhang, T. Y. Li, G. F. Wang, R. Shahbazian-Yassar, L. B. Hu and C. Wang, Nat. Commun. , 2019, 10 , 4011 CrossRef PubMed .
- W. J. Dai, T. Lu and Y. Pan, J. Power Sources , 2019, 430 , 104–111 CrossRef CAS .
- S. Nellaiappan, N. K. Katiyar, R. Kumar, A. Parui, K. D. Malviya, K. G. Pradeep, A. K. Singh, S. Sharma, C. S. Tiwary and K. Biswas, ACS Catal. , 2020, 10 , 3658–3663 CrossRef CAS .
- S. Guo and C. T. Liu, Prog. Nat. Sci.: Mater. Int. , 2011, 21 , 433–446 CrossRef .
- J. Chen, X. Y. Zhou, W. L. Wang, B. Liu, Y. K. Lv, D. P. Xu, W. Yang and Y. Liu, J. Alloys Compd. , 2019, 785 , 1294 CrossRef CAS .
- K. N. Zhao, X. Li and D. Su, Acta Phys.-Chim. Sin. , 2021, 37 , 2009077 Search PubMed .
- A. Amiri and R. Shahbazian-Yassar, J. Mater. Chem. A , 2021, 9 , 782–823 RSC .
- X. H. Yan and Y. Zhang, Scr. Mater. , 2020, 187 , 188–193 CrossRef CAS .
- G. M. Tomboc, T. Kwon, J. Joo and K. Lee, J. Mater. Chem. A , 2020, 8 , 14844–14862 RSC .
- B. Yaakobi, T. R. Boehly, D. D. Meyerhofer, T. J. B. Collins, B. A. Remington, P. G. Allen, S. M. Pollaine, H. E. Lorenzana and J. H. Eggert, Phys. Rev. Lett. , 2005, 95 , 075501 CrossRef CAS PubMed .
- Z. Y. Lv, X. J. Liu, B. Jia, H. Wang, Y. Wu and Z. P. Lu, Sci. Rep. , 2016, 6 , 34213 CrossRef CAS PubMed .
- Y. G. Yao, Z. N. Huang, P. F. Xie, S. D. Lacey, R. J. Jacob, H. Xie, F. J. Chen, A. M. Nie, T. C. Pu, M. Rehwoldt, D. W. Yu, M. R. Zachariah, C. Wang, R. Shahbazian-Yassar, J. Li and L. B. Hu, Science , 2018, 359 , 1489–1494 CrossRef CAS PubMed .
- S. Peulon and D. Lincot, Adv. Mater. , 1996, 8 , 166–170 CrossRef CAS .
- J. E. Dick, C. Renault, B. K. Kim and A. J. Bard, Angew. Chem., Int. Ed. , 2014, 53 , 11859–11862 CrossRef CAS PubMed .
- K. Huang, B. W. Zhang, J. S. Wu, T. Y. Zhang, D. D. Peng, X. Cao, Z. Zhang, Z. Li and Y. Z. Huang, J. Mater. Chem. A , 2020, 8 , 11938–11947 RSC .
- A. Villa, D. Wang, D. S. Su and L. Prati, Catal. Sci. Technol. , 2015, 5 , 55–68 RSC .
- E. D. Bojesen and B. B. Iversen, Crystengcomm , 2016, 18 , 8332–8353 RSC .
- S. J. Gao, S. Y. Hao, Z. N. Huang, Y. F. Yuan, S. Han, L. C. Lei, X. W. Zhang, R. Shahbazian-Yassar and J. Lu, Nat. Commun. , 2020, 11 , 2016 CrossRef CAS PubMed .
- E. V. Shevchenko, D. V. Talapin, H. Schnablegger, A. Kornowski, O. Festin, P. Svedlindh, M. Haase and H. Weller, J. Am. Chem. Soc. , 2003, 125 , 9090–9101 CrossRef CAS PubMed .
- M. Bondesgaard, N. L. N. Broge, A. Mamakhel, M. Bremholm and B. B. Iversen, Adv. Funct. Mater. , 2019, 29 , 1905933 CrossRef CAS .
- C. J. Wang, X. R. Bao, G. W. Du, Y. Wang, K. Chen, M. L. Shen and L. Z. Wang, Int. Urol. Nephrol. , 2014, 46 , 1609–1617 CrossRef CAS PubMed .
- N. L. N. Broge, M. Bondesgaard, F. Sondergaard-Pedersen, M. Roelsgaard and B. B. Iversen, Angew. Chem., Int. Ed. , 2020, 59 , 21920–21924 CrossRef CAS PubMed .
- M. Y. Rekha, N. Mallik and C. Srivastava, Sci. Rep. , 2018, 8 , 8737 CrossRef CAS PubMed .
- S. H. Zhou, G. S. Jackson and B. Eichhorn, Adv. Funct. Mater. , 2007, 17 , 3099–3104 CrossRef CAS .
- S. Alayoglu and B. Eichhorn, J. Am. Chem. Soc. , 2008, 130 , 17479–17486 CrossRef CAS PubMed .
- M. M. Liu, Z. H. Zhang, F. Okejiri, S. Z. Yang, S. H. Zhou and S. Dai, Adv. Mater. Interfaces , 2019, 6 , 1900015 CrossRef CAS .
- Q. L. Li, H. L. Li, V. G. Pol, I. Bruckental, Y. Koltypin, J. Calderon-Moreno, I. Nowik and A. Gedanken, New J. Chem. , 2003, 27 , 1194–1199 RSC .
- K. S. Suslick and G. J. Price, Annu. Rev. Mater. Sci. , 1999, 29 , 295–326 CrossRef CAS .
- S. H. Joo, J. W. Bae, W. Y. Park, Y. Shimada, T. Wada, H. S. Kim, A. Takeuchi, T. J. Konno, H. Kato and I. V. Okulov, Adv. Mater. , 2020, 32 , 1906160 CrossRef CAS PubMed .
- Z. Y. Jin, J. Lv, H. L. Jia, W. H. Liu, H. L. Li, Z. H. Chen, X. Lin, G. Q. Xie, X. J. Liu, S. H. Sun and H. J. Qiu, Small , 2019, 15 , 1904180 CrossRef CAS PubMed .
- C. X. Xu, R. Y. Wang, Y. Zhang and Y. Ding, Nanoscale , 2010, 2 , 906–909 RSC .
- H. J. Qiu, G. Fang, Y. R. Wen, P. Liu, G. Q. Xie, X. J. Liu and S. H. Sun, J. Mater. Chem. A , 2019, 7 , 6499–6506 RSC .
- M. M. Biener, J. Biener, A. Wichmann, A. Wittstock, T. F. Baumann, M. Baumer and A. V. Hamza, Nano Lett. , 2011, 11 , 3085–3090 CrossRef CAS PubMed .
- C. F. Tsai, P. W. Wu, P. Lin, C. G. Chao and K. Y. Yeh, Jpn. J. Appl. Phys. , 2008, 47 , 5755–5761 CrossRef CAS .
- G. W. Yang, Prog. Mater. Sci. , 2007, 52 , 648–698 CrossRef CAS .
- A. V. Kabashin and M. Meunier, J. Appl. Phys. , 2003, 94 , 7941–7943 CrossRef CAS .
- V. Amendola, S. Scaramuzza, F. Carraro and E. Cattaruzza, J. Colloid Interface Sci. , 2017, 489 , 18–27 CrossRef CAS PubMed .
- F. Waag, Y. Li, A. R. Ziefuss, E. Bertin, M. Kamp, V. Duppel, G. Marzun, L. Kienle, S. Barcikowski and B. Gokce, RSC Adv. , 2019, 9 , 18547–18558 RSC .
- P. Wagener, A. Schwenke, B. N. Chichkov and S. Barcikowski, J. Phys. Chem. C , 2010, 114 , 7618–7625 CrossRef CAS .
- E. P. George, D. Raabe and R. O. Ritchie, Nat. Rev. Mater. , 2019, 4 , 515–534 CrossRef CAS .
- H. Q. Song, F. Y. Tian, Q. M. Hu, L. Vitos, Y. D. Wang, J. Shen and N. X. Chen, Phys. Rev. Mater. , 2017, 1 , 023404 CrossRef .
- B. Fultz, Prog. Mater. Sci. , 2010, 55 , 247–352 CrossRef CAS .
- J. W. Yeh, JOM , 2013, 65 , 1759–1771 CrossRef CAS .
- J. W. Yeh, Ann. Chimie Sci. Matériaux , 2006, 31 , 633–648 CrossRef CAS .
- F. Y. Tian, Front. Mater. , 2017, 4 , 36 CrossRef .
- B. Cantor, I. T. H. Chang, P. Knight and A. J. B. Vincent, Mater. Sci. Eng., A , 2004, 375 , 213–218 CrossRef .
- Y. Pei, G. B. Zhou, N. Luan, B. N. Zong, M. H. Qiao and F. Tao, Chem. Soc. Rev. , 2012, 41 , 8140–8162 RSC .
- J. F. Deng, H. X. Li and W. J. Wang, Catal. Today , 1999, 51 , 113–125 CrossRef CAS .
- K. V. Yusenko, S. Riva, P. A. Carvalho, M. V. Yusenko, S. Arnaboldi, A. S. Sukhikh, M. Hanfland and S. A. Gromilov, Scr. Mater. , 2017, 138 , 22–27 CrossRef CAS .
- M. H. Tsai and J. W. Yeh, Mater. Res. Lett. , 2014, 2 , 107–123 CrossRef .
- Y. F. Kao, T. J. Chen, S. K. Chen and J. W. Yeh, J. Alloys Compd. , 2009, 488 , 57–64 CrossRef CAS .
- S. Ranganathan, Curr. Sci. , 2003, 85 , 1404–1406 Search PubMed .
- Y. J. Zhou, Y. Zhang, Y. L. Wang and G. L. Chen, Appl. Phys. Lett. , 2007, 90 , 181904 CrossRef .
- S. Singh, N. Wanderka, B. S. Murty, U. Glatzel and J. Banhart, Acta Mater. , 2011, 59 , 182–190 CrossRef CAS .
- K. Zhao, X. X. Xia, H. Y. Bai, D. Q. Zhao and W. H. Wang, Appl. Phys. Lett. , 2011, 98 , 141913 CrossRef .
- R. N. Singh, A. Singh and Anindita, Int. J. Hydrogen Energy , 2009, 34 , 2052–2057 CrossRef CAS .
- Z. Qi, H. R. Geng, X. G. Wang, C. C. Zhao, H. Ji, C. Zhang, J. L. Xu and Z. H. Zhang, J. Power Sources , 2011, 196 , 5823–5828 CrossRef CAS .
- S. Kasatikov, A. Fantin, A. M. Manzoni, S. Sakhonenkov, A. Makarova, D. Smirnov, E. O. Filatova and G. Schumacher, J. Alloys Compd. , 2021, 857 , 157597 CrossRef CAS .
- G. L. Zhang, K. S. Ming, J. L. Kang, Q. Huang, Z. J. Zhang, X. R. Zheng and X. F. Bi, Electrochim. Acta , 2018, 279 , 19–23 CrossRef CAS .
- K. Y. Tsai, M. H. Tsai and J. W. Yeh, Acta Mater. , 2013, 61 , 4887–4897 CrossRef CAS .
- Q. F. He and Y. Yang, Front. Mater. , 2018, 5 , 42 CrossRef .
- K. Chen, S. Kim, R. Rajendiran, K. Prabakar, G. Z. Li, Z. C. Shi, C. Y. Jeong, J. Kang and O. L. Li, J. Colloid Interface Sci. , 2021, 582 , 977–990 CrossRef CAS PubMed .
- C. F. Tsai, K. Y. Yeh, P. W. Wu, Y. F. Hsieh and P. Lin, J. Alloys Compd. , 2009, 478 , 868–871 CrossRef CAS .
- A. L. Wang, H. C. Wan, H. Xu, Y. X. Tong and G. R. Li, Electrochim. Acta , 2014, 127 , 448–453 CrossRef CAS .
- J. Barranco and A. R. Pierna, J. Non-Cryst. Solids , 2008, 354 , 5153–5155 CrossRef CAS .
- X. T. Chen, C. H. Si, Y. L. Gao, J. Frenzel, J. Z. Sun, G. Eggeler and Z. H. Zhang, J. Power Sources , 2015, 273 , 324–332 CrossRef CAS .
- S. K. Wu, Y. Pan, N. Wang, T. Lu and W. J. Dai, Int. J. Miner., Metall. Mater. , 2019, 26 , 124–132 CrossRef CAS .
- D. S. Wu, K. Kusada, T. Yamamoto, T. Toriyama, S. Matsumura, I. Gueye, O. Seo, J. Kim, S. Hiroi, O. Sakata, S. Kawaguchi, Y. Kubota and H. Kitagawa, Chem. Sci. , 2020, 11 , 12731–12736 RSC .
- C. S. Zhou, R. C. Bowman, Z. Z. Fang, J. Lu, L. Xu, P. Sun, H. Liu, H. Wu and Y. Liu, ACS Appl. Mater. Interfaces , 2019, 11 , 38868–38879 CrossRef CAS PubMed .
- M. M. Shi, D. Bao, S. J. Li, B. R. Wulan, J. M. Yan and Q. Jiang, Adv. Energy Mater. , 2018, 8 , 1800124 CrossRef .
- S. Y. Li, X. W. Tang, H. L. Jia, H. L. Li, G. Q. Xie, X. J. Liu, X. Lin and H. J. Qiu, J. Catal. , 2020, 383 , 164–171 CrossRef CAS .
- Y. G. Yao, Z. N. Huang, T. Y. Li, H. Wang, Y. F. Liu, H. S. Stein, Y. M. Mao, J. L. Gao, M. L. Jiao, Q. Dong, J. Q. Dai, P. F. Xie, H. Xie, S. D. Lacey, I. Takeuchi, J. M. Gregoire, R. Z. Jiang, C. Wang, A. D. Taylor, R. Shahbazian-Yassar and L. B. Hu, Proc. Natl. Acad. Sci. U. S. A. , 2020, 117 , 6316–6322 CrossRef CAS PubMed .
- S. A. Kube and J. Schroers, Scr. Mater. , 2020, 186 , 392–400 CrossRef CAS .
- C. J. H. Jacobsen, S. Dahl, B. S. Clausen, S. Bahn, A. Logadottir and J. K. Norskov, J. Am. Chem. Soc. , 2001, 123 , 8404–8405 CrossRef CAS PubMed .
- P. M. Forster, J. Eckert, B. D. Heiken, J. B. Parise, J. W. Yoon, S. H. Jhung, J. S. Chang and A. K. Cheetham, J. Am. Chem. Soc. , 2006, 128 , 16846–16850 CrossRef CAS PubMed .
- K. S. Suslick, S. B. Choe, A. A. Cichowlas and M. W. Grinstaff, Nature , 1991, 353 , 414–416 CrossRef CAS .
- H. Zahn and J. Kramer, Z. Phys. , 1933, 86 , 413–420 CrossRef CAS .
- H. I. Schlesinger, H. C. Brown, A. E. Finholt, J. R. Gilbreath, H. R. Hoekstra and E. K. Hyde, J. Am. Chem. Soc. , 1953, 75 , 215–219 CrossRef CAS .
- J. Vanwonterghem, S. Morup, C. J. W. Koch, S. W. Charles and S. Wells, Nature , 1986, 322 , 622–623 CrossRef .
- Z. Li, J. Y. Fu, Y. Feng, C. K. Dong, H. Liu and X. W. Du, Nat. Catal. , 2019, 2 , 1107–1114 CrossRef CAS .
- A. Molnar, G. V. Smith and M. Bartok, Adv. Catal. , 1989, 36 , 329–383 CAS .
- H. Li, K. Shin and G. Henkelman, J. Chem. Phys. , 2018, 149 , 174705 CrossRef PubMed .
- J. W. Yeh, S. K. Chen, J. Y. Gan, S. J. Lin, T. S. Chin, T. T. Shun, C. H. Tsau and S. Y. Chang, Metall. Mater. Trans. A , 2004, 35a , 2533–2536 CrossRef CAS .
- D. F. Wu, J. C. Zhou and Y. D. Li, AIChE J. , 2007, 53 , 2618–2629 CrossRef CAS .
- D. F. Wu, L. Y. Song, B. Q. Zhang and Y. D. Li, Chem. Eng. Sci. , 2003, 58 , 3995–4004 CrossRef CAS .
- V. Stamenkovic, B. S. Mun, K. J. J. Mayrhofer, P. N. Ross, N. M. Markovic, J. Rossmeisl, J. Greeley and J. K. Norskov, Angew. Chem., Int. Ed. , 2006, 45 , 2897–2901 CrossRef CAS PubMed .
- A. J. Medford, A. Vojvodic, J. S. Hummelshoj, J. Voss, F. Abild-Pedersen, F. Studt, T. Bligaard, A. Nilsson and J. K. Norskov, J. Catal. , 2015, 328 , 36–42 CrossRef CAS .
- F. Abild-Pedersen, J. Greeley, F. Studt, J. Rossmeisl, T. R. Munter, P. G. Moses, E. Skulason, T. Bligaard and J. K. Norskov, Phys. Rev. Lett. , 2007, 99 , 016105 CrossRef CAS PubMed .
- W. C. Sheng, M. Myint, J. G. G. Chen and Y. S. Yan, Energy Environ. Sci. , 2013, 6 , 1509–1512 RSC .
- D. Voiry, H. Yamaguchi, J. W. Li, R. Silva, D. C. B. Alves, T. Fujita, M. W. Chen, T. Asefa, V. B. Shenoy, G. Eda and M. Chhowalla, Nat. Mater. , 2013, 12 , 850–855 CrossRef CAS PubMed .
- M. Mavrikakis, B. Hammer and J. K. Norskov, Phys. Rev. Lett. , 1998, 81 , 2819–2822 CrossRef .
- J. R. Kitchin, J. K. Norskov, M. A. Barteau and J. G. Chen, Phys. Rev. Lett. , 2004, 93 , 156801 CrossRef CAS PubMed .
- J. Hwang, R. R. Rao, L. Giordano, Y. Katayama, Y. Yu and Y. Shao-Horn, Science , 2017, 358 , 751–756 CrossRef CAS PubMed .
- X. L. Tian, X. Zhao, Y. Q. Su, L. J. Wang, H. M. Wang, D. Dang, B. Chi, H. F. Liu, E. J. M. Hensen, X. W. Lou and B. Y. Xia, Science , 2019, 366 , 850–856 CrossRef CAS PubMed .
- B. Hammer and J. K. Norskov, Surf. Sci. , 1995, 343 , 211–220 CrossRef CAS .
- L. A. Kibler, A. M. El-Aziz, R. Hoyer and D. M. Kolb, Angew. Chem., Int. Ed. , 2005, 44 , 2080–2084 CrossRef CAS PubMed .
- M. C. Luo and S. J. Guo, Nat. Rev. Mater. , 2017, 2 , 17059 CrossRef CAS .
- S. Schnur and A. Gross, Phys. Rev. B: Condens. Matter Mater. Phys. , 2010, 81 , 033402 CrossRef .
- K. Yan, T. A. Maark, A. Khorshidi, V. A. Sethuraman, A. A. Peterson and P. R. Guduru, Angew. Chem., Int. Ed. , 2016, 55 , 6175–6181 CrossRef CAS PubMed .
- H. T. Wang, S. C. Xu, C. Tsai, Y. Z. Li, C. Liu, J. Zhao, Y. Y. Liu, H. Y. Yuan, F. Abild-Pedersen, F. B. Prinz, J. K. Norskov and Y. Cui, Science , 2016, 354 , 1031–1036 CrossRef CAS PubMed .
- L. Wang, Z. H. Zeng, W. P. Gao, T. Maxson, D. Raciti, M. Giroux, X. Q. Pan, C. Wang and J. Greeley, Science , 2019, 363 , 870–874 CrossRef CAS PubMed .
- M. Escudero-Escribano, P. Malacrida, M. H. Hansen, U. G. Vej-Hansen, A. Velazquez-Palenzuela, V. Tripkovic, J. Schiotz, J. Rossmeisl, I. E. L. Stephens and I. Chorkendorff, Science , 2016, 352 , 73–76 CrossRef CAS PubMed .
- P. Strasser, S. Koh, T. Anniyev, J. Greeley, K. More, C. F. Yu, Z. C. Liu, S. Kaya, D. Nordlund, H. Ogasawara, M. F. Toney and A. Nilsson, Nat. Chem. , 2010, 2 , 454–460 CrossRef CAS PubMed .
- M. Asano, R. Kawamura, R. Sasakawa, N. Todoroki and T. Wadayama, ACS Catal. , 2016, 6 , 5285–5289 CrossRef CAS .
- N. Todoroki, Y. Iijima, R. Takahashi, Y. Asakimori and T. Wadayama, J. Electrochem. Soc. , 2013, 160 , F591–F596 CrossRef CAS .
- I. E. L. Stephens, A. S. Bondarenko, U. Gronbjerg, J. Rossmeisl and I. Chorkendorff, Energy Environ. Sci. , 2012, 5 , 6744–6762 RSC .
- V. Jalan and E. J. Taylor, J. Electrochem. Soc. , 1983, 130 , 2299–2301 CrossRef CAS .
- H. Zhang, Y. Pan, Y. Z. He and H. S. Jiao, Appl. Surf. Sci. , 2011, 257 , 2259–2263 CrossRef CAS .
- J. Li, Q. H. Fang, B. Liu and Y. Liu, Acta Mater. , 2018, 147 , 35–41 CrossRef CAS .
- Z. Q. Fu, W. P. Chen, H. M. Wen, D. L. Zhang, Z. Chen, B. L. Zheng, Y. Z. Zhou and E. J. Lavernia, Acta Mater. , 2016, 107 , 59–71 CrossRef CAS .
- B. Gludovatz, A. Hohenwarter, K. V. S. Thurston, H. B. Bei, Z. G. Wu, E. P. George and R. O. Ritchie, Nat. Commun. , 2016, 7 , 10602 CrossRef CAS PubMed .
- S. Y. Liu and Y. J. Wei, Extreme Mechanics Letters , 2017, 11 , 84–88 CrossRef .
- A. J. Zaddach, C. Niu, C. C. Koch and D. L. Irving, JOM , 2013, 65 , 1780–1789 CrossRef CAS .
- M. Kim, C. Lee, S. M. Ko and J. M. Nam, J. Solid State Chem. , 2019, 270 , 295–303 CrossRef CAS .
- K. Jiang, H. X. Zhang, S. Z. Zou and W. B. Cai, Phys. Chem. Chem. Phys. , 2014, 16 , 20360–20376 RSC .
- T. Bligaard and J. K. Norskov, Electrochim. Acta , 2007, 52 , 5512–5516 CrossRef CAS .
- C. M. Clausen, T. A. A. Batchelor, J. K. Pedersen and J. Rossmeisl, Adv. Sci. , 2021, 8 , 2003357 CrossRef CAS PubMed .
- L. Luo, Z. Y. Duan, H. Li, J. Kim, G. Henkelman and R. M. Crooks, J. Am. Chem. Soc. , 2017, 139 , 5538–5546 CrossRef CAS PubMed .
- Y. W. Wang, W. J. Qiu, E. H. Song, F. Gu, Z. H. Zheng, X. L. Zhao, Y. Q. Zhao, J. J. Liu and W. Q. Zhang, Natl. Sci. Rev. , 2018, 5 , 327–341 CrossRef CAS .
- C. T. Campbell, Acc. Chem. Res. , 2019, 52 , 984–993 CrossRef CAS PubMed .
- F. Z. Song, W. Li and Y. J. Sun, Inorganics , 2017, 5 , 40 CrossRef .
- F. Z. Song, W. Li, J. Q. Yang, G. Q. Han, T. Yan, X. Liu, Y. Rao, P. L. Liao, Z. Cao and Y. J. Sun, ACS Energy Lett. , 2019, 4 , 1594–1601 CrossRef CAS .
- T. Loffler, A. Savan, H. Meyer, M. Meischein, V. Strotkotter, A. Ludwig and W. Schuhmann, Angew. Chem., Int. Ed. , 2020, 59 , 5844–5850 CrossRef PubMed .
- J. K. Norskov, T. Bligaard, A. Logadottir, J. R. Kitchin, J. G. Chen, S. Pandelov and J. K. Norskov, J. Electrochem. Soc. , 2005, 152 , J23–J26 CrossRef CAS .
- J. D. Benck, T. R. Hellstern, J. Kibsgaard, P. Chakthranont and T. F. Jaramillo, ACS Catal. , 2014, 4 , 3957–3971 CrossRef CAS .
- W. Li, D. H. Xiong, X. F. Gao and L. F. Liu, Chem. Commun. , 2019, 55 , 8744–8763 RSC .
- J. K. Norskov, J. Rossmeisl, A. Logadottir, L. Lindqvist, J. R. Kitchin, T. Bligaard and H. Jonsson, J. Phys. Chem. B , 2004, 108 , 17886–17892 CrossRef CAS .
- V. Viswanathan, H. A. Hansen, J. Rossmeisl and J. K. Norskov, ACS Catal. , 2012, 2 , 1654–1660 CrossRef CAS .
- V. R. Stamenkovic, B. S. Mun, M. Arenz, K. J. J. Mayrhofer, C. A. Lucas, G. F. Wang, P. N. Ross and N. M. Markovic, Nat. Mater. , 2007, 6 , 241–247 CrossRef CAS PubMed .
- J. Greeley, I. E. L. Stephens, A. S. Bondarenko, T. P. Johansson, H. A. Hansen, T. F. Jaramillo, J. Rossmeisl, I. Chorkendorff and J. K. Norskov, Nat. Chem. , 2009, 1 , 552–556 CrossRef CAS PubMed .
- Z. Y. Duan and G. F. Wang, Phys. Chem. Chem. Phys. , 2011, 13 , 20178–20187 RSC .
- T. A. A. Batchelor, J. K. Pedersen, S. H. Winther, I. E. Castelli, K. W. Jacobsen and J. Rossmeisl, Joule , 2019, 3 , 834–845 CrossRef CAS .
- K. D. Jensen, J. Tymoczko, J. Rossmeisl, A. S. Bandarenka, I. Chorkendorff, M. Escudero-Escribano and I. E. L. Stephens, Angew. Chem., Int. Ed. , 2018, 57 , 2800–2805 CrossRef CAS PubMed .
- K. D. Gilroy, A. Ruditskiy, H. C. Peng, D. Qin and Y. N. Xia, Chem. Rev. , 2016, 116 , 10414–10472 CrossRef CAS PubMed .
- M. K. Debe, Nature , 2012, 486 , 43–51 CrossRef CAS PubMed .
- H. A. Gasteiger and N. M. Markovic, Science , 2009, 324 , 48–49 CrossRef CAS PubMed .
- I. E. L. Stephens, J. Rossmeisl and I. Chorkendorff, Science , 2016, 354 , 1378–1379 CrossRef CAS PubMed .
- J. K. Pedersen, T. A. A. Batchelor, D. X. Yan, L. E. J. Skjegstad and J. Rossmeisl, Curr. Opin. Electrochem. , 2021, 26 , 100651 CrossRef CAS .
- Z. L. Lu, Z. W. Chen and C. V. Singh, Matter , 2020, 3 , 1318–1333 CrossRef .
- A. Logadottir, T. H. Rod, J. K. Norskov, B. Hammer, S. Dahl and C. J. H. Jacobsen, J. Catal. , 2001, 197 , 229–231 CrossRef CAS .
- T. Bligaard, J. K. Norskov, S. Dahl, J. Matthiesen, C. H. Christensen and J. Sehested, J. Catal. , 2004, 224 , 206–217 CrossRef CAS .
- Y. Z. Shi, B. Yang and P. K. Liaw, Metals , 2017, 7 , 43 CrossRef .
- B. Song, Y. Yang, M. Rabbani, T. T. Yang, K. He, X. B. Hu, Y. F. Yuan, P. Ghildiyal, V. P. Dravid, M. R. Zachariah, W. A. Saidi, Y. Z. Liu and R. Shahbazian-Yassar, ACS Nano , 2020, 14 , 15131–15143 CrossRef PubMed .
- C. F. Wise and J. M. Mayer, J. Am. Chem. Soc. , 2020, 142 , 12544–12545 CrossRef CAS PubMed .
- H. M. Sun, Z. H. Yan, F. M. Liu, W. C. Xu, F. Y. Cheng and J. Chen, Adv. Mater. , 2020, 32 , 1806326 CrossRef CAS PubMed .
- Y. L. Zhu, Q. Lin, Y. J. Zhong, H. A. Tahini, Z. P. Shao and H. T. Wang, Energy Environ. Sci. , 2020, 13 , 3361–3392 RSC .
- T. X. Nguyen, Y. H. Su, C. C. Lin, J. Ruan and J. M. Ting, Adv. Sci. , 2021, 8 , 2002446 CrossRef CAS PubMed .
- H. Chen, Y. F. Sun, S. Z. Yang, H. Wang, W. Dmowski, T. Egami and S. Dai, Chem. Commun. , 2020, 56 , 15056–15059 RSC .
To revisit this article, visit My Profile, then View saved stories .
- What Is Cinema?
Nickel Boys Is a Bold Experiment in Perspective

The new film Nickel Boys , opening the New York Film Festival on September 27 after a debut in Telluride, is an adaptation of Colson Whitehead ’s Pultizer-winning novel, a fictionalized account of a real Florida-panhandle reform school whose staff, for decades, abused and in some cases murdered its charges—particularly the Black boys sent there ostensibly for rehabilitation. It is sorrowful, damning subject matter, of the sort that might merit sprawling cinematic treatment.
But director RaMell Ross , a photographer turned documentarian now making his scripted feature debut, takes an entirely different tack in approaching this hefty material. His film is shot mostly in first-person perspective; as an unlucky teenager, Elwood ( Ethan Herisse ), finds himself dragged into this swampy hell, we watch what unfolds through his eyes. Peripheral vision is limited. Ross tunnels into the heart of a helpless tragedy in narrow framing; we understand the forces bearing down on Elwood, but we rarely see them in full.
Nickel Boys is the most formally inventive of its fall-movie-season brethren, a bold swing of a literary adaptation that mostly earns its gimmick. Though really, gimmick is a cheap word for what Ross is doing. The nervy technical conceit of Nickel Boys has an instructive purpose; it is not mere “look what I can do” grandstanding. Ross has designed his film as a plunge into the visceral realities of racism in America, with no outside framing offering even a hint of escape.
It’s a tough film, rigorous in its intent. But Ross does allow for grace, comfort, quotidian beauty. The opening stretches of his film show us Elwood’s pre-incarceration life in all its precarious potential: he’s a promising student, doted on by the grandmother who raised him ( Aunjanue Ellis-Taylor ), in puppy-dog love with a girlfriend, grasping in happy amazement at a future rushing toward him. It’s the mid-1960s in Florida and Elwood is a young Black man, so he is certainly faced with daunting obstacles. But he has, out of both passion and principled intellect, decided to stand on the front lines of the struggle he shares with so many. This is an engaged, determined kid, a portrait crafted in flickering mosaic.
Even if Elwood were on a less noble track, though, that would in no way justify the harsh punishment he receives when he unwittingly hitches a ride in a stolen car, is picked up by the police, and is remanded to Nickel Academy. Ross is careful to insist that no child, of any background, deserves such routine dehumanization. But yes: we are meant to particularly mourn for the future stolen from Elwood, who followed all the rules and was nonetheless relegated to brutal subjugation. Nickel Boys is a stark reflection of our country’s gruesome record; even after emancipation, Black men routinely found themselves dragged into a kind of slavery different only in name.
Ross has made a busy collage of a film, jumping between evocative image and plot with a swiftness that sometimes leaves the audience on the outside. We are not really left to sit in any one particular experience—either of joy or confinement—long enough for it to fully seep in. Ross’s stuttering poetry is more considered than simply stylish, but is not quite as emotionally enveloping as more traditional narrative might be.
Ross does not hew strictly to his central device. The film breaks away from Elwood’s gaze on occasion to show us that of his fellow prisoner Turner (the remarkable Brandon Wilson ), a more rebellious free-spirit than his quiet and cowed friend. Stitched together, Elwood and Turner’s perspectives create an intimate picture of ragged and wary optimism. Deliverance must be possible, and yet it cannot be assumed.
The details of Elwood and Turner’s hardship are sometimes directly confronted. More often than not, though, they are only alluded to. Ross insightfully, shrewdly renders how such a nightmare might be processed, especially by someone so young and naive as Elwood. The danger is close by, but life also must plug along. It is only in hindsight, maybe, that the scope of what happened—to Elwood, to everyone—can truly be grasped. Ross makes room for that recollection, drifting into the future (shooting from behind actor Daveed Diggs ’s head) to show what adulthood looks like for men struggling to reclaim the humanity robbed of them as teenagers.
Nickel Boys closes in walloping fashion: a final series of sensory flashes serves as heartbreaking coda. One wonders how much more might be felt had Ross laid things out more linearly, had he taken the time to truly delve into particular moments, to flesh out the dimensions of Elwood and Turner’s daily life.
Still, Ross’s elegant, thorough re-creation of time and place is plenty effective as is. And anyway, is witnessing explicit trauma really any more valuable than what Ross chooses to show us? Nickel Boys is perhaps a rebuke to the idea that violence must be plainly stated in order to be understood. Here, it is palpably present in every negative space. What Ross instead affords these young men is the dignity of a point of view, drawing the viewer into the bracing immediacy of mind and body. Nickel Boys is an arduous—and also humane and lovely—trek in another’s shoes. It’s an immersion into two lives that evokes the histories of countless more.
More Great Stories From Vanity Fair
Ta-Nehisi Coates on Efforts to Ban Between the World and Me
The Wild, True Tale Behind Monsters: The Lyle and Erik Menendez Story
Why Princess Diana “Ripped the Corset Out” of Her Iconic Met Gala Gown
MAGA Mega-Donor Tim Mellon Is Rocking the Family Boat
Life After The Prick : An Exclusive Peek at Bad Sisters Season 2
The Reported RFK Jr.–Olivia Nuzzi “Relationship” Casts New Scrutiny on All Journalists
How Mariah Carey Became the Queen of Christmas
33 Fall TV Shows We Can’t Wait to Watch
Team Harris vs. Team Trump: A Running List of Celebrity Endorsements
From the Archive: Dominick Dunne’s Menendez Trial Courtroom Notebook

Richard Lawson
Chief critic.

COMMENTS
Isaac Newton Eyes Optics. Experimental result: The nature of color and light. When: 1665-1666. Before he was that Isaac Newton — scientist extraordinaire and inventor of the laws of motion, calculus and universal gravitation (plus a crimefighter to boot) — plain ol' Isaac found himself with time to kill.
Pavlov's Dog: And 49 Other Experiments That Revolutionised Psychology by Adam Hart-Davies, Elwin Street, 2018. A very quick run through of a few more famous scientific experiments. Opening Skinner's Box: Great Psychological Experiments of the 20th Century by Lauren Slater, Bloomsbury, 2005/2016.
3. Bobo Doll Experiment Study Conducted by: Dr. Alburt Bandura. Study Conducted between 1961-1963 at Stanford University . Experiment Details: During the early 1960s a great debate began regarding the ways in which genetics, environmental factors, and social learning shaped a child's development. This debate still lingers and is commonly referred to as the Nature vs. Nurture Debate.
3: Henry Cavendish weighs the world (1798) In Cambridge, England, one of the world's greatest physics laboratories is named for Henry Cavendish, an 18th-century scientist who weighed the world. Quite an undertaking, you might think! Actually, Cavendish's famous experiment involved measuring the density of Earth, from which its mass (or weight ...
2. Freeze Water Instantly. When purified water is cooled to just below freezing point, a quick nudge or an icecube placed in it is all it takes for the water to instantly freeze. You can finally ...
10 Cool Chemistry Experiments. ThoughtCo / Hilary Allison. Chemistry is king when it comes to making science cool. There are many interesting and fun projects to try, but these 10 chemistry experiments might be the coolest. Whether you want to witness color transformations with copper and nitric acid or create a foam spectacle with hydrogen ...
Musical Jars Science Experiment. This super easy experiment is simple as it is fun! Kids make their own musical instruments with clear jars and water then investigate sound waves, pitch, and more. When the experiment is complete, use the colorful new "instrument" for a fun music lesson. Kids can play and take turns to "name that tune"!
Go Science Kids. 43. "Flip" a drawing with water. Light refraction causes some really cool effects, and there are multiple easy science experiments you can do with it. This one uses refraction to "flip" a drawing; you can also try the famous "disappearing penny" trick.
10 Scientific Discoveries That Changed The World. DNA, gravity, and germ theory are a few of the key findings in history that forever shifted the course of human civilization. Learn how these scientific discoveries changed the world. By Allison Futterman and Monica Cull. Jan 18, 2024 7:30 AMJan 18, 2024 8:27 AM.
What Makes Ice Melt Fastest? Build a Paper Roller Coaster. Elephant Toothpaste. Make Popping Boba Balls Out of Your Drinks. Make the Wind Work for You! Tallest Paper Tower Challenge. How to Turn a Potato Into a Battery. Bath Bomb Science. Make Ice Cream in a Bag.
They have made great strides in lifting that veil of mystery. In addition to providing us with food for stimulating party conversations, some of the most famous psychological experiments of the last century reveal surprising and universal truths about nature. ... 10. Rosenbaum's experiment. Among the most interesting investigations in this ...
Here are the top 10 greatest physics experiments of all time that have shed light on some of the mysteries that surround us. 1. Galileo Galilei's Experiment on Speed of Falling Objects. Before Galileo, Aristotle had argued that heavy objects fall at a faster rate than lighter objects. But Galileo who is famed for his work on gravity, motion ...
10 Great Experiments That Contribute to Modern Psychology 1. Milgram's Experiment: In the Milgram experiment, a subject's willingness to administer a certain amount of shock served as a proxy for obedience. Even though several of the subjects became quite anxious, upset, and hostile toward the researcher, they nevertheless obeyed ...
With that in mind, here are our top 20 picks for the BEST science experiments to do at home with your kids! Baking Soda and Vinegar Experiments. There are so many cool experiments you can do with these pantry staples. Yes, you can do a standard volcano, but you can also hatch dino eggs, create fireworks, set off explosive bottle rockets and more!
Simple Science Experiments with Baking Soda and Vinegar. Baking soda + vinegar = a great chemical reaction! This fizzy reaction can fuel a variety of simple science experiments at home. First of all, we have tested and found out the absolute best combination of baking soda and vinegar to get the best reaction possible.
#10: Blood Model in a Jar. Teaches Kids About: Human biology; Difficulty Level: Easy; Messiness Level: Medium; This blood model experiment is a great way to get kids to visual what their blood looks like and how complicated it really is. Each ingredient represents a different component of blood (plasma, platelets, red blood cells, etc.), so you ...
Thermite Reaction. The thermite reaction is one of the more dramatic chemistry experiments. All you do is mix a metal and a metal oxide and ignite it. But, this is no ordinary fire. The reaction is very bright and extremely hot. It is the burning of metal, so it serves as an example of oxidation, combustion, and exothermic reactions.
These experiments make great family projects to do together, too - much better than vegetating in front of the TV! Below you will find a selection of science experiments spanning biology, chemistry, physics and Earth sciences. Each section will list the equipment you need, some instructions, and an explanation of what happens. ...
Here's a list of over 30 Science Fair ideas to get you started. Then download science experiments, and watch experiment videos to inspire your project.
The human family tree expanded significantly in the past decade, with fossils of new hominin species discovered in Africa and the Philippines. The decade began with the discovery and ...
Wash and dry your hands. Leave the eggs in the glasses for 12 hours. After 12 hours, remove the eggs from the glasses of soda one at a time. Rinse them in cool water and pat them dry with the ...
AS SEEN ON THE ELLEN DEGENERES SHOW! · 50 fantastic experiments from bestselling author Steve Spangler · All experiments vetted by the scientists at the Smithsonian Institution · Step-by-step instructions with full color photos Smithsonian 10-Minute Science Experiments gives curious young readers dozens of colorful, exciting projects designed to teach them about the basics of science ...
Science experiments you can do at home! Explore an ever growing list of hundreds of fun and easy science experiments. Have fun trying these experiments at home or use them for science fair project ideas. Explore experiments by category, newest experiments, most popular experiments, easy at home experiments, or simply scroll down this page for tons of awesome experiment ideas!
Lu et al. developed a fast-moving bed pyrolysis (FEBP) strategy to synthesize HEA NPs on granular supports including carbon, γ-Al 2 O 3 and zeolite ().This method was conducted at 923 K with a population speed of 20 cm s −1, resulting in the formation of a small size (∼2 nm) of HEA NPs within 5 s.The critical size of HEA nucleus is small enough to reduce excess free energy used for ...
42mm: 46mm: Dimensions: 42 x 36 x 9.7 mm: 46 x 39 x 9.7 mm: IP rating: IP6X: IP6X: Display resolution: 446 x 374 pixels: 496 x 416 pixels: Display size: 1.77 inches: 1.96 inches
"This is the greatest office in the world." Go inside the epic return of Yellowstone. New episodes drop Nov. 10 at 8/7c, only on Paramount Network. #Yellowst...
The new film Nickel Boys, opening the New York Film Festival on September 27 after a debut in Telluride, is an adaptation of Colson Whitehead's Pultizer-winning novel, a fictionalized account of ...