

The Winkler Method - Measuring Dissolved Oxygen
Created by Monica Z. Bruckner, Montana State University

What is the Winkler Method?
The Winkler Method is a technique used to measure dissolved oxygen in freshwater systems. Dissolved oxygen is used as an indicator of the health of a water body, where higher dissolved oxygen concentrations are correlated with high productivity and little pollution. This test is performed on-site, as delays between sample collection and testing may result in an alteration in oxygen content.
How does the Winkler Method Work?
The Winkler Method uses titration to determine dissolved oxygen in the water sample. A sample bottle is filled completely with water (no air is left to skew the results). The dissolved oxygen in the sample is then "fixed" by adding a series of reagents that form an acid compound that is then titrated with a neutralizing compound that results in a color change. The point of color change is called the "endpoint," which coincides with the dissolved oxygen concentration in the sample. Dissolved oxygen analysis is best done in the field, as the sample will be less altered by atmospheric equilibration.
Applications
Dissolved oxygen analysis can be used to determine:
- the health or cleanliness of a lake or stream,
- the amount and type of biomass a freshwater system can support,
- the amount of decomposition occurring in the lake or stream.
How to- Sample Collection, Preparation, Analytical Protocols, and Concerns

- 2ml Manganese sulfate
- 2ml alkali-iodide-azide
- 2ml concentrated sulfuric acid
- 2ml starch solution
- Sodium thiosulfate
These reagents are available in dissolved oxygen field kits, such as those made by the Hach Company. Please use caution when using these reagents, as they can be hazardous to one's health. Procedure:
- Carefully fill a 300-mL glass Biological Oxygen Demand (BOD) stoppered bottle brim-full with sample water.
- Immediately add 2mL of manganese sulfate to the collection bottle by inserting the calibrated pipette just below the surface of the liquid. (If the reagent is added above the sample surface, you will introduce oxygen into the sample.) Squeeze the pipette slowly so no bubbles are introduced via the pipette.
- Add 2 mL of alkali-iodide-azide reagent in the same manner.
- Stopper the bottle with care to be sure no air is introduced. Mix the sample by inverting several times. Check for air bubbles; discard the sample and start over if any are seen. If oxygen is present, a brownish-orange cloud of precipitate or floc will appear. When this floc has settle to the bottom, mix the sample by turning it upside down several times and let it settle again.
- Add 2 mL of concentrated sulfuric acid via a pipette held just above the surface of the sample. Carefully stopper and invert several times to dissolve the floc. At this point, the sample is "fixed" and can be stored for up to 8 hours if kept in a cool, dark place. As an added precaution, squirt distilled water along the stopper, and cap the bottle with aluminum foil and a rubber band during the storage period.
- In a glass flask, titrate 201 mL of the sample with sodium thiosulfate to a pale straw color. Titrate by slowly dropping titrant solution from a calibrated pipette into the flask and continually stirring or swirling the sample water.
- Add 2 mL of starch solution so a blue color forms.
- Continue slowly titrating until the sample turns clear. As this experiment reaches the endpoint, it will take only one drop of the titrant to eliminate the blue color. Be especially careful that each drop is fully mixed into the sample before adding the next. It is sometimes helpful to hold the flask up to a white sheet of paper to check for absence of the blue color.
- The concentration of dissolved oxygen in the sample is equivalent to the number of milliliters of titrant used. Each mL of sodium thiosulfate added in steps 6 and 8 equals 1 mg/L dissolved oxygen.
Results Analysis

- Dissolved Oxygen and Biochemical Oxygen Demand ( more info )
Teaching Activities
- Water What-ifs: Water Quality and Dissolved Oxygen ( more info )
- How to Measure Dissolved Oxygen ( more info )
- Water Quality For Freshwater Organisms ( This site may be offline. )
« Previous Page
- Faculty Resource Center
- Biochemistry
- Bioengineering
- Cancer Research
- Developmental Biology
- Engineering
- Environment
- Immunology and Infection
- Neuroscience
- JoVE Journal
- JoVE Encyclopedia of Experiments
- JoVE Chrome Extension
- Environmental Sciences
- Pharmacology
- JoVE Science Education
- JoVE Lab Manual
- JoVE Business
- Videos Mapped to your Course
- High Schools
- Videos Mapped to Your Course
- 00:00 Overview
- 01:14 Principles of Measuring Dissolved Oxygen in Surface Water
- 03:27 Sample Collection and Fixing in the Field
- 04:42 Measuring Dissolved Oxygen in Surface Water Samples in the Laboratory
- 06:04 Results
- 07:01 Applications
- 09:07 Summary
Dissolved Oxygen in Surface Water
Source: Laboratories of Margaret Workman and Kimberly Frye – Depaul University
Dissolved oxygen (DO) measurements calculate the amount of gaseous oxygen dissolved in surface water, which is important to all oxygen-breathing life in river ecosystems, including fish species preferred for human consumption ( e.g. bluegill and bass), as well as decomposer species critical to the recycling of biogeochemical materials in the system.
The oxygen dissolved in lakes, rivers, and oceans is crucial for the organisms and creatures living in it. As the amount of dissolved oxygen drops below normal levels in water bodies, the water quality is harmed and creatures begin to die. In a process called eutrophication, a body of water can become hypoxic and will no longer be able to support living organisms, essentially becoming a “dead zone.”
Eutrophication occurs when excess nutrients cause algae populations to grow rapidly in an algal bloom. The algal bloom forms dense mats at the surface of the water blocking out two essential inputs of oxygen for water: gas exchange from the atmosphere and photosynthesis in the water due to the lack of light below the mats. As dissolved oxygen levels decline below the surface, oxygen-breathing organisms die-off in large amounts, creating an increase in organic matter. The excess organic matter causes an increase in the oxygen-breathing decomposer populations in the benthic zone, which further depletes the remaining dissolved oxygen levels during the metabolic decomposition activity. Once the oxygen levels become this low, mobile oxygen-breathing species ( e.g. fish) will move away, leaving no aerobic life in the water and creating a dead zone.
The Azide-Winkler titration method uses titration to determine the concentration of an unknown in a sample. Specifically, sodium thiosulfate is used to titrate iodine, which can be stoichiometrically related to the amount of dissolved oxygen in a sample.
The Azide-Winkler method is used to measure DO on site, where surface water is collected. Manganese(II) sulfate and potassium hydroxide are added to the sample, and the dissolved oxygen in the sample oxidizes the manganese and forms a brown precipitate. Azide is added in the form of a purchased alkaline iodide-azide reagent to correct for the presence of nitrites, which are found in wastewater samples and can interfere with the Winkler oxidation procedure.

Sulfuric acid is then added to acidify the solution, and the precipitate dissolves. Under these conditions, the iodide from the alkaline iodide-azide reagent in the solution is converted into iodine.
Thiosulfate is then used to titrate the iodine in the presence of an added starch indicator.
At the endpoint of this titration, the blue solution will turn clear. The amount of dissolved oxygen in the sample is quantified in direct proportion to the amount of thiosulfate required to reach the endpoint.

1. Sample Dissolved Oxygen Measurement
- At the water collection site, use a calibrated pipette to add 2 mL manganous sulfate to a clear 300-mL BOD bottle filled with the sample water. Be careful not to introduce oxygen into the sample by inserting the pipette tip under the sample surface and carefully dispensing manganous sulfate. This will avoid creating bubbles until the sample is “fixed” and prevents change to the dissolved oxygen concentration.
- Using the same technique, add 2 mL alkaline iodide-azide reagent.
- Immediately insert the stopper, tilting the bottle slightly and quickly pushing the stopper in place so no air bubbles are trapped in the bottle.
- Carefully invert several times (without creating air bubbles) to mix. A floccule (floc) will form from a precipitated aggregation of material with a cloudy appearance ( Figure 1 ).
- Wait until the floc in the solution has settled. Again, invert the bottle several times and wait until the floc has settled. The sample is now fixed to prevent change in dissolved oxygen content, and can be transported back to the lab and stored for up to 8 h, if needed, in a cool and dark condition.
- If storing, samples should be sealed using a small amount of deionized water squirted around the stopper, and the stopper should be wrapped in aluminum foil, secured with a rubber band.
- Pipette 2 mL of concentrated sulfuric acid into the sample by holding the pipette tip just above the sample surface. Invert carefully several times to dissolve the floc ( Figure 2 ).
- In a glass flask, and using a calibrated pipette, titrate 200 mL of sample water with 0.025 N standardized sodium thiosulfate, swirling and mixing continuously until a pale straw color forms ( Figure 3 ).
- Add 2 mL of starch indicator solution with a dropper and swirl to mix. Once the starch Indicator is added, the solution will turn blue ( Figure 4 ).
- Continue the titration, adding one drop at a time until one drop dissipates the blue, causing the colorless endpoint. Be sure to add each drop of titrant carefully and to evenly mix each drop before adding the next. Holding the sample against a white piece of paper can help enhance visualization of the endpoint.
- The concentration of DO is equivalent to the volume (mL) of titrant used. Each milliliter of sodium thiosulfate added to the water sample equals 1 mg/L dissolved oxygen.

Dissolved oxygen is crucial for river and lake ecosystems to support aerobic life. The Azide-Winkler titration method allows quantification of the amount of dissolved oxygen in surface water samples.
Gaseous oxygen dissolved in surface water is required for the survival of the organisms living in it; decomposers critical to recycling of biogeochemical materials in the ecosystem, or fish species preferred for human consumption. As oxygen levels fall below normal in water systems, water quality is harmed and organisms begin to die.
The Azide-Winkler titration method is a standard test to determine the concentration of dissolved oxygen in a sample. Sodium thiosulfate is used to titrate iodine, which is stochiometrically related to the amount of dissolved oxygen in the sample.
This video will illustrate the principles behind dissolved oxygen quantification, the process of performing the Azide-Winker titration, and the interpretation of dissolved oxygen measures.
Eutrophication is the introduction of excess nutrients into an ecosystem. This causes algae populations to grow rapidly into dense mats, known as algal blooms. These mats can lead to hypoxia, or low oxygen levels, by blocking out gas exchange at the surface, and prevent photosynthesis by blocking sunlight. Oxygen breathing organisms begin to die, causing an increase in organic matter, which in turn causes an increase in oxygen dependent decomposers, depleting oxygen resources yet further. Finally, mobile oxygen-dependent organisms move away, leaving a dead zone with no aerobic life.
To test the level of dissolved oxygen in a water source, the Azide-Winkler method can be used to measure dissolved oxygen directly in the field, or samples can be fixed and taken to the laboratory for further analysis.
Manganese sulfate and potassium hydroxide are added to the sample, forming manganese hydroxide. This reduces the dissolved oxygen, forming a brown precipitate. Alkaline iodide-azide reagent is added to correct for the presence of nitrates found in wastewater samples that can interfere with the oxidation procedure.
Added sulfuric acid acidifies the solution and dissolves the precipitate. This new compound oxidizes the iodide from the alkaline iodine-azide reagent to iodine.
Next, a starch indicator is added that will turn blue in the presence of iodine. Thiosulfate, which turns iodine back into iodide, is used to titrate the iodine. When the titration is complete, the blue solution will turn colorless. The amount of dissolved oxygen in the sample is proportional to the amount of thiosulfate required to turn the solution from blue to colorless.
Now that we are familiar with the principles behind measuring dissolved oxygen in water samples, let’s take a look at how this is carried out in the field and the laboratory.
The experiment will begin at the collection site. First, collect the sample water in a clear 300-mL BOD bottle. Next, measure and record the temperature of water from the water source. Carefully add 2 mL manganous sulfate to the sample by inserting the pipette tip under the water surface and slowly dispense to avoid creating bubbles.
Using the same technique, add 2 mL alkaline iodine-azide reagent, and immediately insert the stopper, tilting the bottle slightly so no air is trapped in the bottle.
Carefully invert several times to mix the solution, taking care not to create air bubbles. A precipitate will form, causing a cloudy appearance. Let the precipitate in the solution settle, and then mix thoroughly by inverting the bottle several times before letting it settle again. Samples should be sealed using a small amount of deionized water squirted around the stopper, then wrapped in aluminum foil and secured with a rubber band. The sample is now fixed, and can be transported back to the laboratory.
Once the samples have been fixed, they are transported to the lab for further analysis. First, holding the pipette tip just above the sample surface, add 2 mL of concentrated sulfuric acid into the sample. Invert several times to dissolve the precipitate. Using a glass flask and calibrated pipette, titrate 200 mL of the pre-treated sample water with 0.025 N standardized sodium thiosulfate, swirling and mixing continuously until a pale straw color forms.
Once the solution is straw colored, add 2, 1-mL droplets of starch indicator solution and swirl to mix. The solution will turn blue. Continue the titration, adding one drop of sodium thiosulfate at a time and mixing slowly using a stir bar until the blue dissipates and the solution becomes colorless. Hold the sample against a white piece of paper to enhance visualization. Record the volume of thiosulfate added.
The concentration of dissolved oxygen is proportional to the volume of sodium thiosulfate added to the sample. Each milliliter added is equivalent to 1 mg/L, or parts per million, dissolved oxygen.
The maximum amount of oxygen that can be dissolved in water varies by water temperature. Dissolved oxygen measurements in mg/L are converted to percent saturation using water temperature and a conversion chart. Saturation of 91 to 110% dissolved oxygen is considered excellent; between 71 and 90% is good, 51-70% is fair, and below 50% is poor.
Dissolved oxygen levels of 6 mg/L are sufficient to support most aquatic species. Levels below 4 mg/L are stressful to the majority of aquatic animals, so biodiversity will be affected. Water containing less than 2 mg/L dissolved oxygen will not support aerobic aquatic life.
The ability to quantify the amount of dissolved oxygen in a water source also has alternative methods, and many relevant practical applications. Some of these are explored here.
Dissolved oxygen and temperature can also be measured using a handheld LabQuest monitor with dissolved oxygen and temperature probes. For dissolved oxygen, plug the probe into channel 1. Units should be in mg/L. Submerge the probe into the water sample, circulating the probe slowly through the sample to avoid consuming oxygen in a localized area. When the readings appear to stabilize, record the value.
Most fish require moderate to good levels of dissolved oxygen in their habitats to thrive and reproduce. For fish farms, which may occupy man-made or natural lakes or streams, being able to test dissolved oxygen levels can help farm managers to choose a good initial set-up site, or to keep track of the health of their pools or streams.
Monitoring dissolved oxygen can also be useful for habitat management and conservation. If a lake or river region contains protected or endangered flora or fauna, monitoring of dissolved oxygen levels can give an indication of the health of the ecosystem. If levels change rapidly, this could indicate danger for the protected species, and may indicate that a management intervention strategy should be implemented.
The United States Environmental Protection Agency, the EPA, suggests a number of measures to correct dissolved oxygen levels in ecosystems. These include correct and minimal use of fertilizers, proper wastewater treatment, not discharging sewage from boats, and preserving adjoining rivers, streams, and wetlands. Reducing nitrogen oxides by minimizing electricity and automobile use and choosing more efficient boat engines can also help to maintain appropriate dissolved oxygen levels in water resources.
You’ve just watched JoVE’s introduction to measuring dissolved oxygen in surface waters. You should now understand the principles behind dissolved oxygen measurement, how to quantify dissolved oxygen in your own water samples, and how to interpret your findings and their implications for the environment. Thanks for watching!
A dissolved oxygen level of 6 mg/L is sufficient for most aquatic species. Dissolved oxygen levels below 4 mg/L are stressful to most aquatic animals. Dissolved oxygen levels below 2 mg/L will not support aerobic aquatic life ( Figure 5 ).
The maximum amount of oxygen that can be dissolved in water varies by temperature ( Table 1 ).
DO measurements in mg/L are converted to % saturation using water temperature and the conversion chart below ( Figure 6 ).
DISSOLVED OXYGEN LEVELS (% SATURATION) Excellent: 91 – 110 Good: 71 – 90 Fair: 51 – 70 Poor: < 50

Table 1. Maximum amounts of oxygen that can be dissolved in water by temperature.
Applications and Summary
Slow-moving rivers are particularly vulnerable to low DO levels, and in extreme cases, these DO levels can lead to hypoxic conditions, creating “dead zones” where aerobic life is no longer supported by a body of water ( Figure 7 ). Once plants and animals die-off, the build-up of sediment that occurs can also raise the riverbed, allowing plants to colonize over the water and could lead to the loss of the river all together ( Figure 8 ). Surface waters at higher altitudes are also more vulnerable to low DO levels, as atmospheric pressure decreases with increasing altitude, and less oxygen gas is suspended in the water.
Low DO levels support life forms considered unappealing or unfit for human use, including leeches and aquatic worms ( Oligochaeta) .

Related Videos

Turbidity and Total Solids in Surface Water

Nutrients in Aquatic Ecosystems

Water Quality Analysis via Indicator Organisms
- Country / Region Change
Contáctenos
Encuéntranos
Methods for Measuring Dissolved Oxygen Levels
Dissolved oxygen levels can be measured by a basic chemical analysis method (titration method), an electrochemical analysis method (diaphragm electrode method), and a photochemical analysis method (fluorescence method). The diaphragm electrode method is the most widely used method.
- Titration Method
- Diaphragm Electrode Method
- Fluorescence method
- The Story of pH
- The Story of ORP
- The Story of Conductivity
- The Story of Ion
- The Story of Salt
- The Story of Dissolved Oxygen
- The Basis of pH
- The Basis of Conductivity
- The Basis of Ion
- The Basis of ORP
- The Basis of DO Measurement
- Measurement of pH
- Measurement of Conductivity
- Measurement of Ion
- Measurement of ORP

# Type at least 1 character to search # Hit enter to search or ESC to close

No products in the cart.
Product Categories
- New Products
- Dissolved Oxygen
- Conductivity
Temperature
- Oxygen (gas)
- Atlas Iot - Raspberry Pi Software
- AtlasDesktop
- Dosing Pumps
- Color Sensing
- Flow Meters
- Float Switches
- Probe Mounting
- EZO-Complete
- Waterproofing
- Carrier Boards
- Calibration Solutions
- Electrical Isolation
- Embedded Solutions
- EZO™ Accessories
Featured Products

- Distributors
- Calculators
- Privacy Policy
- Return Policy
How To Test Dissolved Oxygen In Water
- September 14, 2021

Share This Post
Testing dissolved oxygen (DO) in water is either measured via chemical analysis such as a titrimetric method, electroanalytical (using galvanic & polarographic probes), optical dissolved oxygen, and colorimetric methods. However, modern techniques mainly use electrochemical or optical sensor methods.
Oxygen is not only important in the air we breathe, but it is also an essential element in liquids like water which is why it is important to test dissolved oxygen (DO).
DO is the measurement of the number of free oxygen molecules in water. Measuring DO levels is an important indicator in industries such as water quality systems and aquatic ecosystems as oxygen is an essential chemical element for most forms of life.
In this article we will look at why testing DO is so important and the best ways to measure DO levels in water.
Testing Dissolved Oxygen (DO) In Water
DO is measured using a Dissolved Oxygen meter . The best time to measure DO in water is the same time every day, as concentrations can fluctuate throughout the day. DO is usually measured in milligrams per liter (mg/L) or percentage saturation (% sat), but sometimes it can be measured in parts per million (ppm), which allows measurement comparisons between sites that have different salinity and temperature values.
We can test DO in water through the following methods: titrimetric, electroanalytical (galvanic & polarographic probes), optical dissolved oxygen, and colorimetric.
Titrimetric Method
Titrations use one liquid where the concentration is already determined (titrant), to identify the concentration of another (your sample).
Iodemtry titrations use iodine as an indicator; the iodine indicator will either appear or disappear at the end of the titration. Titrations for testing DO in water are known as the “Winkler Method”. The Winkler Method gives you a “one-time measurement” of the sample being tested.
When using the Winkler Method , water samples are collected, fixed, and titrated in the field or a laboratory setting. As atmospheric contact and agitation can shift DO levels, you must fix the sample with the reagents immediately. Titration methods use a specialized bottle (BOD bottle) that seals without trapping air inside. Usually, all reagents come pre-measured to make it easier and increase accuracy in testing DO in water. To get an accurate DO reading, ensure the titrant solution is proportional to the sample you are testing.
The Winkler Method is still commonly used to test DO in water, however, there are some concerns with inaccuracies, possible sample contaminants/interferences, and human error. That is why new technologies have created easier and more accurate ways to test DO in water.

Electroanyalytical (Galvanic & Polarographic Probes) Method
If you are testing in the lab or the field, this is probably the easiest way to test DO in water. Electroanalytical or electrochemical dissolved oxygen sensors are also known as amperometric or Clark-type Sensors.
There are two types of electroanalytical sensors: galvanic and polarographic probes. The probes work off redox (oxidation-reduction) reactions, providing continuous and live measurements. As both probes have an applied voltage, they require a “warm-up time” before use to polarize the electrodes before measuring the DO in water.
Galvanic Probes/Sensors
These are membrane probes that have two parts that produce a voltage, acting as a battery (the metals have different electrode potentials). A thin semi-permeable membrane inside the cap of the electrode allows gasses to pass through and block anything else. When oxygen diffuses across the membrane, it dissolves into the probe cap that contains a buffered electrolyte. This allows the oxygen to react with the cathode (usually silver) in the electrode, gaining an electron. The electron given to the oxygen molecule comes from the anode (usually zinc or lead) in the electrode, creating a voltage between the anode and cathode in the probe. It is when this current is formed the meter can convert the reading taken from the probe into a DO concentration value.
Because of self-polarization, these sensors do not require a warm-up time.
Polarographic Probes/Sensors
These work slightly differently from galvanic probes, but, polarographic probes also contain a thin semi-permeable membrane allowing oxygen into an unbuffered electrolyte. However, instead of acting like a battery, a voltage is applied between the silver anode and gold cathode in the probe. The voltage acts as a catalyst driving an oxygen reaction. When oxygen hits the cathode, an electron is added creating a current, determining the DO concentration.
Polarographic probes can be separated further into steady-state and rapid-pulsing sensors.
Steady-state sensors allow you to measure DO in water without having to stir the sample. When using a Rapid-pulsing sensor , there is also no need to stir the sample, but it contains a third silver electrode as these sensors turn on and off every few seconds to allow the DO to replenish on the cathode surface when it reaches the membrane. Both still utilize a cathode and anode and measure DO by creating a constant voltage to polarize the electrons.

Optical Dissolved Oxygen Method
This method also uses a probe with a semi-permeable membrane to test DO in water, but the probe and meter monitor luminescence instead of monitoring a reaction. Sometimes these DO meters are called fluorescent sensors. However, this is technically incorrect as the probes emit blue light not UV (ultraviolet) light.
The probe emits the blue light that excites (electrons gain energy) light-sensitive material inside the probe cap. When it becomes relaxed (reaches its normal energy state) it emits a red light that is measured as it hits the light sensor inside the probe; the red light is reflected by the dye. If DO is present in water, it suppresses the red light as wavelengths are limited/altered. The frequency, intensity, and decay of the red light are dependent on the amount of DO in the water.
While optical dissolved oxygen probes provide a continuous measurement of DO, they can be affected by humidity.
Colorimetric Method
This method measures color and comes in two variations: Indigo Carmine and the Rhodazine D method. Chemical reagents are added that react with DO in the sample to display a particular color. The chemical reagents used are similar to the modern Winkler Method. How intense the color is, is proportional to how much DO is in the sample.
Indigo Carmine is used for measuring DO concentrations between 0.2 and 15 ppm, whereas Rhodazine D is used to measure much lower DO concentrations (ppb).
Indigo Carmine produces a blue color where the intensity is proportional to the DO concentration. If using this method, keep reagents away from bright lighting, as this can deteriorate the Indigo Carmine. This method is not affected by salinity, temperature, or dissolved gases, but ferric iron, and nitrate and sodium sulfate can. Results are obtained between 30 seconds (low-range tests) and 2-minutes (high-range tests).
Rhodazine D reagents react with DO, producing a rose-colored or pink solution. Oxidizing agents (chlorine, cupric copper, and ferric iron) can interfere with results creating higher DO readings, however, this method is not affected by salinity or sulfide that are usually present in water samples. As this method is time-dependent, ensure you analyze the water sample within 30 seconds of adding the reagent.
Either a spectrophotometer, colorimeter, or simple comparator can be used to measure DO in water using the colorimetric method.

Why Is It Important To Test Dissolved Oxygen (DO) In Water?
Testing DO in water depends on the industry. For example, you may need to test DO when brewing beer or measure the dissolved oxygen in wastewater .
Dissolved oxygen is an essential parameter in monitoring water quality and a key indicator of healthy aquatic ecosystems. Low DO levels in water are problematic for most aquatic life, often creating dead zones where aquatic life dies off.
In wastewater treatments, testing DO levels in water helps us understand the biodegradable organic matter and the biological oxygen demand (BOD). Both these tests indicate general water quality.
Alternatively, too much oxygen in water can also be harmful, this is known as supersaturated oxygen. DO in water originates from the atmosphere and photosynthesis, which can be affected by temperature, salinity, and atmospheric pressure.
What Can Affect Dissolved Oxygen (DO) In Water?
DO concentrations are affected by temperature, salinity, pressure, and humidity, so you will need to take this into account when testing DO.
Temperature is one of the biggest, if not the most, common factors that directly affects DO in water. Colder water contains more oxygen than warmer water, as particle motion decreases. As particles get more excited and bounce around more, they collide and break the bonds that hold them together.
Therefore, the lower the DO, the higher the temperature, and inversely the DO concentration increases as temperature decreases.
Salinity can also affect how much DO is in water. Freshwater contains more oxygen than saltwater because of the charge a salt molecule carries. Salt molecules are attracted to water molecules and easily dissolved in water. If salt is present, oxygen cannot attract to water molecules, therefore as salinity levels increase in a solution, DO decreases.
Atmospheric pressure
When we talk about pressure and DO, we are referring to atmospheric pressure. As atmospheric pressure decreases, the partial pressure of oxygen also decreases, therefore, the concentration of DO increases. So, as altitude or atmospheric pressure increases, the number of DO molecules absorbed in water decreases as there is less pressure forcing the oxygen to be diffused in the water, increasing the partial pressure of oxygen.
Water vapor or humidity is another factor that is often not thought about, yet it has major implications for DO concentrations, and can also affect the calibration of some DO meters.
When humidity levels increase, the partial pressure of oxygen increases, which also increases the level of DO.
Which Instrument Is the Best For Measuring Dissolved Oxygen (DO) In Water?
Now that you have an understanding of testing DO in water and why it is important, you may be thinking which equipment is best for testing. First, you need to think about accessibility, will you need a portable meter or will a bench top meter work better for you?
Depending on where you wish to test DO in water, there are two types used: portable meters or benchtop meters.
Portable meters provide flexibility to test wherever you want while still receiving high-level accurate readings.
They either use the colorimetric method, optical DO probe, or the electroanalytical method using galvanic or polarographic probes. Choosing which portable meter you need depends on the sample being measured, the level of accuracy you require, and your personal preference. Some portable meters can test more water parameters than DO so always do your research beforehand to get the right meter for you.

When it comes to electrochemical sensing and measuring DO in water, it can get confusing, which is why we offer a variety of meters to meet your testing needs. Whether you are measuring a simple sample or are working with a PLC, we have you covered when it comes to dissolved oxygen probes . All our portable meters allow you to take highly accurate and interference-free readings of DO in water.
Benchtop meters also come in a variety of types meeting your testing requirements. One thing you need to consider with benchtop meters is space. Some benchtop meters can take up more space than electroanalytical probes. However, there are some benchtop meters that have a “zero-footprint” allowing you to mount them to a wall.
Summing Up How To Test Dissolved Oxygen (DO) In Water & DO Testing Equipment
Dissolved oxygen is an important characteristic of water quality in many industries (hydroponics, food and beverage industries, aquariums, environmental sampling, wastewater, etc.).
Testing dissolved oxygen in water is either measured via chemical analysis such as a titrimetric method, electroanalytical (using galvanic & polarographic probes), optical dissolved oxygen, and colorimetric methods. However, modern techniques mainly use electrochemical probes.
If you would like to learn more about other water quality measurements, characteristics, or applications for DO, do not hesitate to contact our world-class team at Atlas Scientific .
Dissolved Oxygen Probes & Sensor

Subscribe To Our Newsletter
Get product updates and learn from the best, more to explore.

Chlorine In Drinking Water: Pros, Cons, And Frequently Asked Questions
Chlorine in drinking water is a topic that often stirs up heated discussions. Chlorine is commonly used as a disinfectant in public water supplies to kill harmful bacteria and other microbes. This safeguards our health from waterborne diseases. However, the downside is that chlorine in drinking water can also have potential health risks if the

Understanding The Relationship Between pH And Electrical Conductivity
Electrical conductivity (EC) and pH are two fundamental properties that are closely related. The relationship between the two is complex, yet typically, as pH increases, the conductivity increases in a solution. This is because an increase in pH leads to an increase in the ion concentration, thus increasing the electrical conductivity. Electrical conductivity (EC) and
Want to learn more about our products?
Atlas scientific | all rights reserved © 2024.
To track your order please enter your Order ID in the box below and press the "Track" button. This was given to you on your receipt and in the confirmation email you should have received.
Billing email

An official website of the United States government
Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

- Digg
Latest Earthquakes | Chat Share Social Media
Dissolved Oxygen and Water
Water properties photo gallery, learn about water properties through pictures, water properties information by topic, water quality information by topic, water science school home.
- Publications
Dissolved oxygen (DO) is a measure of how much oxygen is dissolved in the water - the amount of oxygen available to living aquatic organisms. The amount of dissolved oxygen in a stream or lake can tell us a lot about its water quality.
• Water Science School HOME • Water Properties topics • Water Quality topics •

The USGS has been measuring water for decades. Some measurements, such as temperature , pH , and specific conductance are taken almost every time water is sampled and investigated, no matter where in the U.S. the water is being studied. Another common measurement often taken is dissolved oxygen (DO), which is a measure of how much oxygen is dissolved in the water - DO can tell us a lot about water quality.
Although water molecules contain an oxygen atom, this oxygen is not what is needed by aquatic organisms living in natural waters. A small amount of oxygen, up to about ten molecules of oxygen per million of water, is actually dissolved in water. Oxygen enters a stream mainly from the atmosphere and, in areas where groundwater discharge into streams is a large portion of streamflow, from groundwater discharge. This dissolved oxygen is breathed by fish and zooplankton and is needed by them to survive.
Dissolved oxygen and water quality

Rapidly moving water, such as in a mountain stream or large river, tends to contain a lot of dissolved oxygen, whereas stagnant water contains less. Bacteria in water can consume oxygen as organic matter decays. Thus, excess organic material in lakes and rivers can cause eutrophic conditions, which is an oxygen-deficient situation that can cause a water body to "die." Aquatic life can have a hard time in stagnant water that has a lot of rotting, organic material in it, especially in summer (the concentration of dissolved oxygen is inversely related to water temperature ), when dissolved-oxygen levels are at a seasonal low. Water near the surface of the lake– the epilimnion– is too warm for them, while water near the bottom–the hypolimnion– has too little oxygen. Conditions may become especially serious during a period of hot, calm weather, resulting in the loss of many fish. You may have heard about summertime fish kills in local lakes that likely result from this problem.
(Source: A Citizen's Guide to Understanding and Monitoring Lakes and Streams )
Dissolved oxygen, temperature, and aquatic life

As the chart shows, the concentration of dissolved oxygen in surface water is affected by temperature and has both a seasonal and a daily cycle. Cold water can hold more dissolved oxygen than warm water. In winter and early spring, when the water temperature is low, the dissolved oxygen concentration is high. In summer and fall, when the water temperature is high, the dissolved-oxygen concentration is often lower.
Dissolved oxygen in surface water is used by all forms of aquatic life; therefore, this constituent typically is measured to assess the "health" of lakes and streams. Oxygen enters a stream from the atmosphere and from groundwater discharge . The contribution of oxygen from groundwater discharge is significant, however, only in areas where groundwater is a large component of streamflow, such as in areas of glacial deposits . Photosynthesis is the primary process affecting the dissolved-oxygen/temperature relation; water clarity and strength and duration of sunlight, in turn, affect the rate of photosynthesis.
Hypoxia and "Dead zones"
You may have heard about a Gulf of Mexico "dead zone" in areas of the Gulf south of Louisiana, where the Mississippi and Atchafalaya Rivers discharge. A dead zone forms seasonally in the northern Gulf of Mexico when subsurface waters become depleted in dissolved oxygen and cannot support most life. The zone forms west of the Mississippi Delta over the continental shelf off Louisiana and sometimes extends off Texas. The oxygen depletion begins in late spring, increases in summer, and ends in the fall.
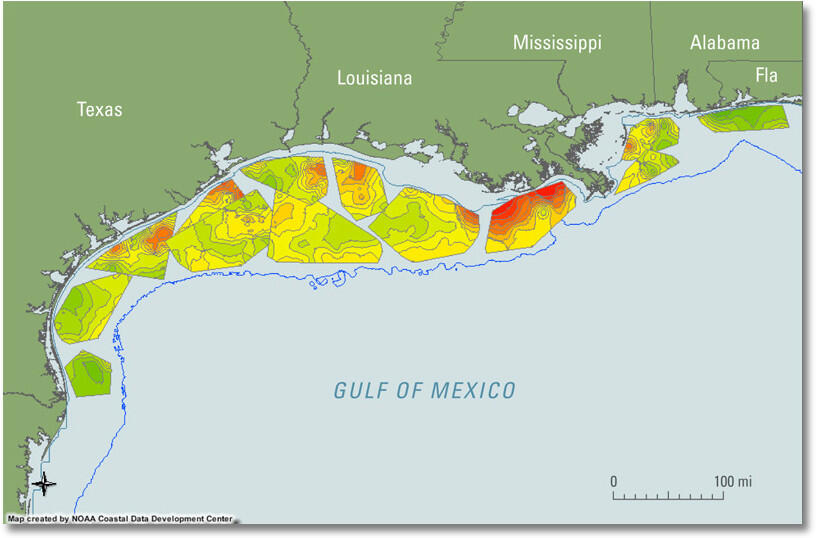
The formation of oxygen-depleted subsurface waters has been associated with nutrient-rich ( nitrogen and phosphorus ) discharge from the Mississippi and Atchafalaya Rivers. Bio-available nutrients in the discharge can stimulate algal blooms, which die and are eaten by bacteria, depleting the oxygen in the subsurface water. The oxygen content of surface waters of normal salinity in the summer is typically more than 8 milligrams per liter (8 mg/L); when oxygen concentrations are less than 2 mg/L, the water is defined as hypoxic (CENR, 2000). The hypoxia kills many organisms that cannot escape, and thus the hypoxic zone is informally known as the “dead zone.”
The hypoxic zone in the northern Gulf of Mexico is in the center of a productive and valuable fishery. The increased frequency and expansion of hypoxic zones have become an important economic and environmental issue to commercial and recreational users of the fishery.
Measuring dissolved oxygen

Field and lab meters to measure dissolved oxygen have been around for a long time. As this picture shows, modern meters are small and highly electronic. They still use a probe, which is located at the end of the cable. Dissolved oxygen is dependent on temperature (an inverse relation), so the meter must be calibrated properly before each use.
Do you want to test your local water quality ?
Water test kits are available from World Water Monitoring Challenge (WWMC), an international education and outreach program that builds public awareness and involvement in protecting water resources around the world. Teachers and water-science enthusiasts: Do you want to be able to perform basic water-quality tests on local waters? WWMC offers inexpensive test kits so you can perform your own tests for temperature , pH , turbidity , and dissolved oxygen .
Do you think you know a lot about water properties? Take our interactive water-properties true/false quiz and test your water knowledge.

Want to know more about dissolved oxygen and water? Follow me to the Nutrients and Eutrophication website!
Learn more about dissolved oxygen and related water topics.

pH and Water


Groundwater Flow and the Water Cycle

Temperature and Water
Conductivity (electrical conductance) and water.

Turbidity and Water

Harmful Algal Blooms (HABs)

Harmful Algae Blooms (HABs)

Transport and Fate of Nutrients
Below are multimedia items associated with dissolved oxygen and related water topics.
Below are publications associated with dissolved oxygen and water.
Chapter A6. Section 6.2. Dissolved oxygen
Gulf of mexico dead zone —the last 150 years.
Jump to main content or area navigation .
- Learn the Issues
- Science & Technology
- Laws & Regulations
Water: Monitoring & Assessment
5.2 Dissolved Oxygen and Biochemical Oxygen Demand
What is dissolved oxygen and why is it important.
The stream system both produces and consumes oxygen. It gains oxygen from the atmosphere and from plants as a result of photosynthesis. Running water, because of its churning, dissolves more oxygen than still water, such as that in a reservoir behind a dam. Respiration by aquatic animals, decomposition, and various chemical reactions consume oxygen.
Wastewater from sewage treatment plants often contains organic materials that are decomposed by microorganisms, which use oxygen in the process. (The amount of oxygen consumed by these organisms in breaking down the waste is known as the biochemical oxygen demand or BOD. A discussion of BOD and how to monitor it is included at the end of this section.) Other sources of oxygen-consuming waste include stormwater runoff from farmland or urban streets, feedlots, and failing septic systems.
Oxygen is measured in its dissolved form as dissolved oxygen (DO). If more oxygen is consumed than is produced, dissolved oxygen levels decline and some sensitive animals may move away, weaken, or die.
DO levels fluctuate seasonally and over a 24-hour period. They vary with water temperature and altitude. Cold water holds more oxygen than warm water (Table 5.3) and water holds less oxygen at higher altitudes. Thermal discharges, such as water used to cool machinery in a manufacturing plant or a power plant, raise the temperature of water and lower its oxygen content. Aquatic animals are most vulnerable to lowered DO levels in the early morning on hot summer days when stream flows are low, water temperatures are high, and aquatic plants have not been producing oxygen since sunset.
| Temperature (°C) | DO (mg/l) | Temperature (°C) | DO (mg/l) | |
| 0 | 14.60 | 23 | 8.56 | |
| 1 | 14.19 | 24 | 8.40 | |
| 2 | 13.81 | 25 | 8.24 | |
| 3 | 13.44 | 26 | 8.09 | |
| 4 | 13.09 | 27 | 7.95 | |
| 5 | 12.75 | 28 | 7.81 | |
| 6 | 12.43 | 29 | 7.67 | |
| 7 | 12.12 | 30 | 7.54 | |
| 8 | 11.83 | 31 | 7.41 | |
| 9 | 11.55 | 32 | 7.28 | |
| 10 | 11.27 | 33 | 7.16 | |
| 11 | 11.01 | 34 | 7.16 | |
| 12 | 10.76 | 35 | 6.93 | |
| 13 | 10.52 | 36 | 6.82 | |
| 14 | 10.29 | 37 | 6.71 | |
| 15 | 10.07 | 38 | 6.61 | |
| 16 | 9.85 | 39 | 6.51 | |
| 17 | 9.65 | 40 | 6.41 | |
| 18 | 9.45 | 41 | 6.41 | |
| 19 | 9.26 | 42 | 6.22 | |
| 20 | 9.07 | 43 | 6.13 | |
| 21 | 8.90 | 44 | 6.04 | |
| 22 | 8.72 | 45 | 5.95 |
Sampling and Equipment Considerations
In contrast to lakes, where DO levels are most likely to vary vertically in the water column, the DO in rivers and streams changes more horizontally along the course of the waterway. This is especially true in smaller, shallower streams. In larger, deeper rivers, some vertical stratification of dissolved oxygen might occur. The DO levels in and below riffle areas, waterfalls, or dam spillways are typically higher than those in pools and slower-moving stretches. If you wanted to measure the effect of a dam, it would be important to sample for DO behind the dam, immediately below the spillway, and upstream of the dam. Since DO levels are critical to fish, a good place to sample is in the pools that fish tend to favor or in the spawning areas they use.
An hourly time profile of DO levels at a sampling site is a valuable set of data because it shows the change in DO levels from the low point just before sunrise to the high point sometime in the midday. However, this might not be practical for a volunteer monitoring program. It is important to note the time of your DO sampling to help judge when in the daily cycle the data were collected.
DO is measured either in milligrams per liter (mg/L) or "percent saturation." Milligrams per liter is the amount of oxygen in a liter of water. Percent saturation is the amount of oxygen in a liter of water relative to the total amount of oxygen that the water can hold at that temperature.
DO samples are collected using a special BOD bottle: a glass bottle with a "turtleneck" and a ground glass stopper. You can fill the bottle directly in the stream if the stream is wadable or boatable, or you can use a sampler that is dropped from a bridge or boat into water deep enough to submerse the sampler. Samplers can be made or purchased. Dissolved oxygen is measured primarily either by using some variation of the Winkler method or by using a meter and probe.
Winkler Method
The Winkler method involves filling a sample bottle completely with water (no air is left to bias the test). The dissolved oxygen is then "fixed" using a series of reagents that form an acid compound that is titrated. Titration involves the drop-by-drop addition of a reagent that neutralizes the acid compound and causes a change in the color of the solution. The point at which the color changes is the "endpoint" and is equivalent to the amount of oxygen dissolved in the sample. The sample is usually fixed and titrated in the field at the sample site. It is possible, however, to prepare the sample in the field and deliver it to a lab for titration.
Dissolved oxygen field kits using the Winkler method are relatively inexpensive, especially compared to a meter and probe. Field kits run between $35 and $200, and each kit comes with enough reagents to run 50 to 100 DO tests. Replacement reagents are inexpensive, and you can buy them already measured out for each test in plastic pillows.
You can also buy the reagents in larger quantities, in bottles, and measure them out with a volumetric scoop. The advantage of the pillows is that they have a longer shelf life and are much less prone to contamination or spillage. The advantage of buying larger quantities in bottles is that the cost per test is considerably less.
The major factor in the expense of the kits is the method of titration they use eyedropper, syringe-type titrator, or digital titrator. Eyedropper and syringe-type titration is less precise than digital titration because a larger drop of titrant is allowed to pass through the dropper opening and, on a micro-scale, the drop size (and thus the volume of titrant) can vary from drop to drop. A digital titrator or a buret (which is a long glass tube with a tapered tip like a pipet) permits much more precision and uniformity in the amount of titrant that is allowed to pass.
If your program requires a high degree of accuracy and precision in DO results, use a digital titrator. A kit that uses an eye dropper-type or syringe- type titrator is suitable for most other purposes. The lower cost of this type of DO field kit might be attractive if you are relying on several teams of volunteers to sample multiple sites at the same time.
Meter and Probe
A dissolved oxygen meter is an electronic device that converts signals from a probe that is placed in the water into units of DO in milligrams per liter. Most meters and probes also measure temperature. The probe is filled with a salt solution and has a selectively permeable membrane that allows DO to pass from the stream water into the salt solution. The DO that has diffused into the salt solution changes the electric potential of the salt solution and this change is sent by electric cable to the meter, which converts the signal to milligrams per liter on a scale that the volunteer can read.
DO meters are expensive compared to field kits that use the titration method. Meter/probe combinations run between $500 and $1,200, including a long cable to connect the probe to the meter. The advantage of a meter/probe is that you can measure DO and temperature quickly at any point in the stream that you can reach with the probe. You can also measure the DO levels at a certain point on a continuous basis. The results are read directly as milligrams per liter, unlike the titration methods, in which the final titration result might have to be converted by an equation to milligrams per liter.
However, DO meters are more fragile than field kits, and repairs to a damaged meter can be costly. The meter/probe must be carefully maintained, and it must be calibrated before each sample run and, if you are doing many tests, in between samplings. Because of the expense, a volunteer program might have only one meter/probe. This means that only one team of samplers can sample DO and they will have to do all the sites. With field kits, on the other hand, several teams can sample simultaneously.
Laboratory Testing of Dissolved Oxygen
If you use a meter and probe, you must do the testing in the field; dissolved oxygen levels in a sample bottle change quickly due to the decomposition of organic material by microorganisms or the production of oxygen by algae and other plants in the sample. This will lower your DO reading. If you are using a variation of the Winkler method, it is possible to "fix" the sample in the field and then deliver it to a lab for titration. This might be preferable if you are sampling under adverse conditions or if you want to reduce the time spent collecting samples. It is also a little easier to titrate samples in the lab, and more quality control is possible because the same person can do all the titrations.
How to collect and analyze samples
The procedures for collecting and analyzing samples for dissolved oxygen consist of the following tasks:
TASK 1 Prepare before leaving for the sampling site
Refer to section 2.3 - Safety Considerations for details on confirming sampling date and time, safety considerations, checking supplies, and checking weather and directions. In addition to the standard sampling equipment and apparel, when sampling for dissolved oxygen, include the following equipment:
If Using the Winkler Method
- Labels for sample bottles
- Field kit and instructions for DO testing
- Enough reagents for the number of sites to be tested
- Kemmerer, Van Dorn, or home-made sampler to collect deep-water samples
- A numbered glass BOD bottle with a glass stopper (1 for each site)
- Data sheet for dissolved oxygen to record results
If Using a Meter and Probe
- DO meter and probe (electrode) (NOTE: Confirm that the meter has been calibrated according to the manufacturer's instructions.)
- Operating manual for the meter and probe
- Extra membranes and electrolyte solution for the probe
- Extra batteries for the meter
- Extension pole
TASK 2 Confirm that you are at the proper location
The directions for sampling should provide specific information about the exact point in the stream from which you are to sample; e.g., "approximately 6 feet out from the large boulder downstream from the west side of the bridge." If you are not sure you are in the exact spot, record a detailed description of where you took the sample so that it can be compared to the actual site later.
TASK 3 Collect samples and fill out the field data sheet
Use a BOD bottle to collect the water sample. The most common sizes are 300 milliliters (mL) and 60 mL. Be sure that you are using the correct volume for the titration method that will be used to determine the amount of DO. There is usually a white label area on the bottle, and this may already be numbered. If so, be sure to record that number on the field data sheet. If your bottle is not already numbered, place a label on the bottle (not on the cap because a cap can be inadvertently placed on a different bottle) and use a waterproof marker to write in the site number.
If you are collecting duplicate samples, label the duplicate bottle with the correct code, which should be determined prior to sampling by the lab supplying the bottles. Use the following procedure for collecting a sample for titration by the Winkler method:
- Remember that the water sample must be collected in such a way that you can cap the bottle while it is still submerged. That means that you must be able to reach into the water with both arms and the water must be deeper than the sample bottle.
- Carefully wade into the stream. Stand so that you are facing one of the banks.
- Collect the sample so that you are not standing upstream of the bottle. Remove the cap of the BOD bottle. Slowly lower the bottle into the water, pointing it downstream, until the lower lip of the opening is just submerged. Allow the water to fill the bottle very gradually, avoiding any turbulence (which would add oxygen to the sample). When the water level in the bottle has stabilized (it won't be full because the bottle is tilted), slowly turn the bottle upright and fill it completely. Keep the bottle under water and allow it to overflow for 2 or 3 minutes to ensure that no air bubbles are trapped.
- Cap the bottle while it is still submerged. Lift it out of the water and look around the "collar" of the bottle just below the bottom of the stopper. If you see an air bubble, pour out the sample and try again.
- Remove the stopper and add the fixing reagents to the sample.
- Immediately insert the stopper so air is not trapped in the bottle and invert several times to mix. This solution is caustic. Rinse your hands if you get any solution on them. An orange-brown flocculent precipitate will form if oxygen is present.
- Wait a few minutes until the floc in the solution has settled. Again invert the bottle several times and wait until the floc has settled. This ensures complete reaction of the sample and reagents. The sample is now fixed, and atmospheric oxygen can no longer affect it. If you are taking the sample to the lab for titration, no further action is necessary. You can store the sample in a cooler for up to 8 hours before titrating it in a lab. If you are titrating the sample in the field, see Task 4: Analyze the Samples.
| Point the bottle downstream and fill gradually. Cap underwater when full. |
Using a DO Meter
If you are using a dissolved oxygen meter, be sure that it is calibrated immediately prior to use. Check the cable connection between the probe and the meter. Make sure that the probe is filled with electrolyte solution, that the membrane has no wrinkles, and that there are no bubbles trapped on the face of the membrane. You can do a field check of the meter's accuracy by calibrating it in saturated air according to th e manufacturer's instructions. Or, you can measure a water sample that is saturated with oxygen, as follows. (NOTE: You can also use this procedure for testing the accuracy of the Winkler method.)
- Fill a l-liter beaker or bucket of tap water. (You may want to bring a gallon jug with water in it for this purpose.) Mark the bottle number as "tap" on the lab sheet.
- Pour this water back and forth into another beaker 10 times to saturate the water with oxygen.
- Use the meter to measure the water temperature and record it in the water temperature column on the field data sheet.
- Find the water temperature of your "tap" sample in Table 5.3. Use the meter to compare the dissolved oxygen concentration of your sample with the maximum concentration at that temperature in the table. Your sample should be within 0.5 mg/L. If it is not, repeat the check and if there is still an error, check the meter's batteries and follow the troubleshooting procedures in the manufacturer's manual.
Once the meter is turned on, allow 15 minute equilibration before calibrating. After calibration, do not turn the meter off until the sample is analyzed. Once you have verified that the meter is working properly, you are ready to measure the DO levels at the sampling site. You might need an extension pole (this can be as simple as a piece of wood) to get the probe to the proper sampling point. Simply secure the probe to the end of the extension pole. A golfer's ball retriever works well because it is collapsible and easy to transport. To use the probe, proceed as follows:
- Place the probe in the stream below the surface.
- Set the meter to measure temperature, and allow the temperature reading to stabilize. Record the temperature on the field data sheet.
- Switch the meter to read dissolved oxygen.
- Record the dissolved oxygen level on the field data sheet.
TASK 4 Analyze the samples
Three types of titration apparatus can be used with the Winkler method: droppers, digital titrators, and burets. The dropper and digital titrator are suited for field use. The buret is more conveniently used in the lab (Fig. 5.8) Volunteer programs are most likely to use the dropper or digital titrator. For titration with a dropper or syringe, which is relatively simple, follow the manufacturer's instructions. The following procedure is for using a digital titrator to determine the quantity of dissolved oxygen in a fixed sample:
| |
- Select a sample volume and sodium thiosulfate titration cartridge for the digital titrator corresponding to the expected dissolved oxygen concentration according to Table 5.4. In most cases, you will use the 0.2 N cartridge and the 100-mL sample volume.
- Insert a clean delivery tube into the titration cartridge.
- Attach the cartridge to the titrator body.
- Hold the titrator with the cartridge tip up. Turn the delivery knob to eject air and a few drops of titrant. Reset the counter to 0 and wipe the tip.
- Use a graduated cylinder to measure the sample volume (from the "fixed" sample in the 300-mL BOD bottle) according to Table 5.4.
- Transfer the sample into a 250-mL Erlenmeyer flask, and place the flask on a magnetic stirrer with a stir bar. If you are in the field, you can manually swirl the flask to mix.
- Place the delivery tube tip into the solution and turn the stirrer on to stir the sample while you're turning the delivery knob.
- Titrate to a pale yellow color.
- Add two dropperfuls of starch indicator solution and swirl to mix. A strong blue color will develop.
- Continue to titrate until the sample is clear. Record the number of digits required. (The color might reappear after standing a few minutes, but this is not a cause for concern. The "first" disappearance of the blue color is considered the endpoint.)
- Calculate mg/L of DO = digits required X digit multiplier (from Table 5.4).
- Record the results in the appropriate column of the data sheet.
Some water quality standards are expressed in terms of percent saturation. To calculate percent saturation of the sample:
- Find the temperature of your water sample as measured in the field.
- Find the maximum concentration of your sample at that temperature as given in Table 5.3.
- Calculate the percent saturation, by dividing your actual dissolved oxygen by the maximum concentration at the sample temperature.
- Record the percent saturation in the appropriate column on the data sheet.
| Expected Range | Sample Volume | Titration Cartridge | Digit Multiplier | |
| 1-5 mg/L | 200 mL | 0.2 N | 0.01 | |
| 2-10 mg/L | 100 mL | 0.2 N | 0.02 | |
| 10+ mg/L | 200 mL | 2.0 N | 0.10 |
TASK 5 Return the samples and the field data sheets to the lab/drop-off point
If you are using the Winkler method and delivering the samples to a lab for titration, double-check to make sure that you have recorded the necessary information for each site on the field data sheet, especially the bottle number and corresponding site nu mber and the times the samples were collected. Deliver your samples and field data sheets to the lab. If you have already obtained the dissolved oxygen results in the field, send the data sheets to your sampling coordinator.
What is biochemical oxygen demand and why is it important?
Biochemical oxygen demand, or BOD, measures the amount of oxygen consumed by microorganisms in decomposing organic matter in stream water. BOD also measures the chemical oxidation of inorganic matter (i.e., the extraction of oxygen from water via chemical reaction). A test is used to measure the amount of oxygen consumed by these organisms during a specified period of time (usually 5 days at 20 C). The rate of oxygen consumption in a stream is affected by a number of variables: temperature, pH, the presence of certain kinds of microorganisms, and the type of organic and inorganic material in the water.
BOD directly affects the amount of dissolved oxygen in rivers and streams. The greater the BOD, the more rapidly oxygen is depleted in the stream. This means less oxygen is available to higher forms of aquatic life. The consequences of high BOD are the same as those for low dissolved oxygen: aquatic organisms become stressed, suffocate, and die.
Sources of BOD include leaves and woody debris; dead plants and animals; animal manure; effluents from pulp and paper mills, wastewater treatment plants, feedlots, and food-processing plants; failing septic systems; and urban stormwater runoff.
Sampling Considerations
BOD is affected by the same factors that affect dissolved oxygen (see above). Aeration of stream water by rapids and waterfalls, for example will accelerate the decomposition of organic and inorganic material. Therefore, BOD levels at a sampling site with slower, deeper waters might be higher for a given volume of organic and inorganic material than the levels for a similar site in highly aerated waters.
Chlorine can also affect BOD measurement by inhibiting or killing the microorganisms that decompose the organic and inorganic matter in a sample. If you are sampling in chlorinated waters, such as those below the effluent from a sewage treatment plant, it is necessary to neutralize the chlorine with sodium thiosulfate. (See APHA, 1992.)
BOD measurement requires taking two samples at each site. One is tested immediately for dissolved oxygen, and the second is incubated in the dark at 20 C for 5 days and then tested for the amount of dissolved oxygen remaining. The difference in oxygen levels between the first test and the second test, in milligrams per liter (mg/L), is the amount of BOD. This represents the amount of oxygen consumed by microorganisms to break down the organic matter present in the sample bottle during the incubation period. Because of the 5-day incubation, the tests should be conducted in a laboratory.
Sometimes by the end of the 5-day incubation period the dissolved oxygen level is zero. This is especially true for rivers and streams with a lot of organic pollution. Since it is not known when the zero point was reached, it is not possible to tell what the BOD level is. In this case it is necessary to dilute the original sample by a factor that results in a final dissolved oxygen level of at least 2 mg/L. Special dilution water should be used for the dilutions. (See APHA, 1992.)
It takes some experimentation to determine the appropriate dilution factor for a particular sampling site. The final result is the difference in dissolved oxygen between the first measurement and the second after multiplying the second result by the dilution factor. More details are provided in the following section.
How to Collect and Analyze Samples
The procedures for collecting samples for BOD testing consist of the same steps described for sampling for dissolved oxygen (see above), with one important difference. At each site a second sample is collected in a BOD bottle and delivered to the lab for DO testing after the 5-day incubation period. Follow the same steps used for measuring dissolved oxygen with these additional considerations:
- Make sure you have two BOD bottles for each site you will sample. The bottles should be black to prevent photosynthesis. You can wrap a clear bottle with black electrician's tape if you do not have a bottle with black or brown glass.
- Label the second bottle (the one to be incubated) clearly so that it will not be mistaken for the first bottle.
- Be sure to record the information for the second bottle on the field data sheet.
The first bottle should be analyzed just prior to storing the second sample bottle in the dark for 5 days at 20 C. After this time, the second bottle is tested for dissolved oxygen using the same method that was used for the first bottle. The BOD i s expressed in milligrams per liter of DO using the following equation:
DO (mg/L) of first bottle - DO (mg/L) of second bottle = BOD (mg/L)
APHA. 1992. Standard methods for the examination of water and wastewater. 18 th ed. American Public Health Association, Washington, DC.
Area Navigation
- Drinking Water
- Education & Training
- Grants & Funding
- Ground Water
- Oceans, Coasts, Estuaries & Beaches
- Rivers & Streams
- Where You Live
- Pollution Prevention & Control
- Resources & Performance
- Water Infrastructure
- What You Can Do
- News by Email
- Privacy and Security Notice
Last updated on Tuesday, March 06, 2012
The Seal of the United States Environmental Protection Agency
Jump to main content.
- Change of Address
- TOS Website

View Issue TOC Volume 34, No. 2 Pages 177 - 183
DIY OCEANOGRAPHY • A Simple and Inexpensive Method for Manipulating Dissolved Oxygen in the Lab
By email: [email protected] affiliation: dauphin island sea lab, dauphin island, al, usa, and university of south alabama, mobile, al, usa search for more papers by this author ">kara j. gadeken and affiliation: dauphin island sea lab, dauphin island, al, usa, and university of south alabama, mobile, al, usa search for more papers by this author ">kelly m. dorgan .
- Published Online: April 22, 2021
- https://doi.org/10.5670/oceanog.2021.202
- Export Article Citation: BibTeX | Reference Manager
BibTeX Citation
Dauphin Island Sea Lab, Dauphin Island, AL, USA, and University of South Alabama, Mobile, AL, USA
Reference Manager Citation
Purpose of device.
Changes in dissolved oxygen concentration can cause dramatic shifts in chemical, biological, and ecological processes in aquatic systems. In shallow coastal areas, this can happen on short timescales, with oxygen increasing during the day due to photosynthesis and declining at night due to respiration. We present a system controlled by an Arduino microprocessor that leverages the oxygen-consuming capacity of sediments to manipulate dissolved oxygen in an aquarium tank to planned concentrations. With minor adjustments to the Arduino code, the system can produce a variety of dissolved oxygen patterns, including a diel cycle. Designed to be user-friendly and scalable if needed, the system uses easily acquired, low-cost electronic and aquarium components. Its simplicity and accessibility permit deeper exploration of the effects of dissolved oxygen variability in aquatic systems, and the use of Arduino code and basic electronics makes it a potential tool for teaching experimental design and instrument fabrication.
The availability of dissolved oxygen (DO) is a major factor governing aquatic ecosystem function and is an indicator of water quality and ecosystem health (Diaz and Rosenberg, 1995, 2011; Wenner et al., 2004; Middelburg and Levin, 2009). The DO concentration in aquatic environments is controlled by the balance of oxygen sources (mixing with the atmosphere, advection of oxygenated water, photosynthetic production) and sinks (aerobic respiration and abiotic oxidation), and shifts in this balance result in cascading chemical, biological, and ecological effects (Middelburg and Levin, 2009). Changes in DO concentration occur across temporal and spatial scales, from widespread, seasons-long bottom hypoxia on continental margins to dramatic daily or sometimes hourly oxygen fluctuations in shallow, semi-enclosed coastal lagoons or embayments. Most low oxygen events are this second type, relatively short in duration but occurring frequently (Wenner et al., 2004). Many researchers have examined the effects of declining or persistent low DO on water and sediment chemistry (McCarthy et al., 2008; Lehrter et al., 2012; Neubacher et al., 2013; Foster and Fulweiler, 2019) and organismal behavior and physiology (Diaz and Rosenberg, 1995; Long et al., 2008; Levin et al., 2009; Sturdivant et al., 2012; Riedel et al., 2014; Calder-Potts et al., 2015). However, it is far less common to see investigations into dynamic variation in DO, likely because of the difficulties in precisely and repeatedly manipulating DO in the lab.
DO can easily be increased in water by bubbling with air, but decreasing DO requires either chemical consumption or physical expulsion of oxygen from solution. An often-used method of decreasing DO involves stripping it from the water by bubbling with N 2 gas. Studies of low oxygen effects that run for multiple weeks or months, however, may require large amounts of N 2 gas, which can be expensive, prompting investigations into ways to reduce the amount of gas needed (Bevan and Kramer, 1988; Peterson and Ardahl, 1992; Grecay and Stierhoff, 2002). Oxygen can also be removed by “vacuum degassing,” applying a partial vacuum to the water to remove DO from solution. This requires an airtight vacuum setup that may not be feasible for some researchers (Mount, 1961; Miller et al., 1994). More recently, researchers have developed methods that rely on chemical consumption rather than physical removal of DO to produce low oxygen water. Thetmeyer et al. (1999) leveraged the respiration of the fish study subject itself to draw down DO, controlling hypoxic, normoxic, and oscillating oxygen treatments with an automated system (Thetmeyer et al., 1999). For this method to work, the fish must consume enough oxygen to change the DO of the experimental environment, which may not be possible for smaller study subjects or those for which wall effects are a concern. Long et al. (2008) presented an alternative method using sediments to decrease DO by percolating water through a “fluidized mud reactor” that consumed oxygen (Long et al., 2008). The resulting anoxic water was then mixed in different proportions with fully oxygenated water to produce predetermined DO concentrations. This setup is convenient for creating water with stable DO concentrations, but it does not easily allow for complex manipulations of DO change through time.
The existing DO manipulation methods pose a barrier to entry for many researchers because of their costs and complexities. Additionally, many methods have been designed to simulate long-term hypoxia, whereas in coastal systems, DO concentrations can vary on short timescales. Here, we describe and test a DO manipulation system that can be constructed in a laboratory or classroom setting using easily acquired electronic and aquarium components. The closed-loop system does not require N 2 gas purging or vacuum degassing; instead, it relies on sediment oxygen demand (SOD) to draw DO down, and it increases DO by periodically opening a solenoid valve to allow oxygenated water to flow in from an upstream reservoir tank ( Figure 1 ). The provided code is uploaded to an Arduino microprocessor that monitors and adjusts the DO in the experiment tank to a pattern planned by the user, simultaneously recording and displaying the DO data. This system was built to study behavior of and SOD by infaunal organisms held individually in small sediment-filled aquaria ( Figure 1 ), but it could be used for a variety of shallow-water systems and study organisms. This simple, low-cost, open-source method of manipulating DO in the lab will allow for more varied studies into how change in DO affects aquatic systems.
| Diagram of oxygen manipulation setup. It is a closed-loop system, constantly cycling water between an oxygenated reservoir tank and a sump. When the dissolved oxygen (DO) in the experiment tank is sensed to be lower than the desired level due to sediment consumption, the solenoid valve is opened, allowing oxygenated water to flow in. A power head in the experiment tank ensures that the water is well mixed, and a layer of bubble wrap floating at the water surface prevents diffusion of atmospheric oxygen into the water. PVC pipe is shown in white, tubing in gray, and wiring in black. Note that, though only one experiment tank is depicted here, several replicate experiment tanks would be needed or several replicate trials should be performed to avoid pseudoreplication. |
MATERIALS AND COSTS
Table 1 outlines the system components. We estimate the total cost of constructing this system to be approximately US$625 in 2021, not including shipping expenses. Note that the components list consists only of consumable items (e.g., wire, tubing, plumbing) and specialized equipment (e.g., Arduino, Atlas Scientific EZO DO kit, pumps, solenoid valve). Non-consumables and tools needed for assembly are not included because it is assumed that the user will already have access to many of these items. Cost of construction might also be substantially lowered if materials can be purchased individually rather than in large packs, or scavenged from other projects, as only a single item or a very small amount is required for most components. An undergraduate or a particularly capable high school student should be able to construct and begin using the system within a couple of weeks.
| Components list for laboratory dissolved oxygen experiments. |
ASSEMBLY STEPS
First, assemble the electronics according to the wiring diagram in Figure 2 . The Arduino Mega®️, solenoid, and relay may all be powered by the same 12V power source. The Adafruit® data logging shield is mounted directly to the Arduino via soldered header pins. The Atlas Scientific®️ EZO DO circuit is mounted on an electrically isolated EZO carrier board and connects to the SCL (clocking) and SDA (data-transmitting) pins on the data logging shield to communicate with the Arduino ( Figure 2 ). Communication between the Arduino and the EZO DO circuit is in I 2 C protocol to allow for easy addition of secondary devices, in case more circuits and sensors are desired to scale up the system. The EZO DO circuit must be converted to I 2 C protocol and the I 2 C address changed to correspond to the address defined in the oxygen manipulation code to communicate with the Arduino. The EZO DO circuit should also be adjusted with temperature and salinity offsets and two-point oxygen calibrated before each use. Directions on conversion to I 2 C protocol, offset adjustments, and calibration are in the EZO DO circuit documentation. An LCD screen is included to display the average measured DO over the previous several measurements and the planned DO, allowing the user to easily assess whether the system is functioning properly and following the prescribed pattern. Power to the LCD can be converted from 12V to 5V DC with a power converter, as shown in Figure 2 , or sourced from the 5V pin on the Arduino. A master on/off power switch is also included and a small push button is wired in to control when the oxygen manipulation code begins (“start” button).
| Wiring diagram. AC power from the grid is converted into DC power, shown as red (VCC) and black (GND) wiring. The VCC terminal block distributes power to each component, and the GND terminal block is a common ground to close the circuit. The Atlas Scientific EZO DO circuit and the LCD screen are controlled via I C protocol from the SCL (clocking) and SDA (data) pins. The Arduino Mega 2560, SD shield, and LCD screen images are from , and the Atlas Scientific EZO DO circuit image is from circuit documentation on . |
When the code is started, it executes in repeated “loops”; within a single loop, the system measures the DO in the tank, calculates the average DO over the last five readings, compares the average DO to the programmed DO, opens the valve to allow in oxygenated water if necessary, logs the data to the SD card, and displays the average and planned DO on the LCD screen. The code may be restarted by pressing the “reset” button on the SD shield and then the wired-in “start” button. The average DO value is used rather than the instantaneous DO value to adjust for inherent noise in measurements (i.e., to prevent the system from allowing a single anomalously low measurement to trigger oxygenated water to flow in, even when the average DO is above the planned level).
Once the electronics have been assembled, construct the closed loop tank system ( Figure 1 ). Three tanks are used: the upstream reservoir tank for oxygenated water, the experiment tank in which study subjects are held and DO is manipulated, and a sump. Oxygenated water will constantly circulate between the reservoir and the sump, and it will be intermittently diverted into the experiment tank whenever DO needs to be increased.
The reservoir tank and the sump may be made from simple plastic bins. Ideally, the experiment tank should be a clear aquarium tank so study subjects may be easily observed. Fit the experiment tank with an outflow standpipe and fill the tank with a layer of organic-rich sediment, which will consume oxygen and drive down DO in the overlying water. Fill the remaining space in the tank up to the top of the standpipe with seawater and allow suspended sediment to settle. Then, make two outflow holes in the reservoir tank. Attach a standpipe to the first hole and add plumbing to the outlet to direct overflow water into the sump. Attach the solenoid valve to the second hole and add piping or tubing to direct flow from the solenoid into the experiment tank. Position the reservoir tank higher than the experiment tank and fill the reservoir tank with seawater up to the standpipe. Place a sump pump in the sump and route tubing from its outflow up to the reservoir tank to close the loop.
Suspend the Atlas Scientific DO sensor in the experiment tank. The water’s surface should be covered by a sheet of bubble wrap (which is oxygen-impermeable and will float at the surface) to prevent diffusion of atmospheric oxygen into the water. A small aquarium power head should be mounted in the experiment tank to gently circulate the water and prevent stagnation. In our setup with a 20 gallon experiment tank, a 120 gph power head was sufficient. Manipulations by the Arduino are based on the readings from the sensor, so it is critical that the water be mixed such that the sensor readings represent the DO of the bulk water in the tank as accurately as possible. It is also important to note that if the system is to be used for rigorous experimental work, having all replicates in one tank presents the issue of pseudoreplication. To resolve this issue, multiple replicate experiment tanks should be plumbed and manipulated “in parallel,” or if it is only feasible to have one experiment tank, multiple replicate trials should be performed over time.
The annotated oxygen manipulation code and the calibration code are freely accessible for download on the code sharing platform GitHub ( https://github.com/kgadeken/OxygenManipulationCode_GadekenDorgan2021 ). It is highly recommended that new users read the code and annotations thoroughly before setting up and using the system.
To serve as a usable method for manipulating oxygen in the lab, the system must reliably, precisely, and accurately produce the programmed DO patterns in the experiment tank. We tested the system by programming it to generate a diel oxygen cycle, with DO concentrations ranging from 3 mg L –1 to 7 mg L –1 . The diel cycle spans a wide range of DO values and demands the system adapt quickly to continually varying rates of DO change, making it a highly rigorous test of the system’s flexibility. Precision was gauged by the difference of each DO measurement from the programmed DO value at that time. To gauge the system’s accuracy, we took corroborating oxygen measurements with an Onset HOBO DO logger. The Atlas Scientific probe and HOBO logger were both two-point calibrated immediately before starting the trial. The Atlas Scientific probe and the HOBO logger were secured in the experiment tank as close together as possible in the upper-middle of the water column at the same vertical height from the sediment surface. The HOBO logger was set to measure DO every five minutes.
Figure 3 shows the results of the diel cycle trial. The system closely followed the diel pattern during rising and high DO periods but deviated slightly during falling DO. This indicates that the sediment was not consuming enough oxygen at these times to keep up with the programmed rate of decrease. At its greatest point, the difference between the measured DO and the planned DO was 0.91 mg L –1 . However, over 99% of the measurements taken deviated from the planned value by less than half of that maximum difference (±0.46 mg L –1 ), and ~89% deviated by less than a quarter (±0.23 mg L –1 ), indicating that the system typically followed the programmed pattern very closely.
| Results of testing of the oxygen manipulation system using a diel oxygen cycle (ranging from 3 mg L to 7 mg L ). Precision was assessed from the difference between the measured DO (blue) and the programmed DO (gray) at each time point. An Onset HOBO DO logger (yellow) was included to take corroborating measurements every five minutes to assess the accuracy of the system’s oxygen measurements and manipulation. |
Because the HOBO was set to take measurements at 5 min intervals while the oxygen manipulation system measured DO every 37 s, values were interpolated from the HOBO sensor measurements to correspond to each of the measurements from the oxygen manipulation system. The HOBO measurements followed the same diel pattern as the system but were positively offset an average of 0.62 mg L –1 . The data from the oxygen manipulation system and the HOBO were both detrended and then analyzed for correlation, and no lag was found between the two data sets. Given this result, it is likely that the difference between the sensor measurements is largely due to calibration error.
A potential issue with this setup lies in the use of sediment oxygen demand as a DO sink. Using SOD rather than N 2 gas limits the rate of oxygen removal from the water, as shown in the diel cycle trial during the periods of DO decline ( Figure 3 ). Vigorous purging with N 2 gas or vacuum degassing can remove all oxygen from solution in seconds or minutes (Mount, 1961), whereas our system using SOD typically takes several hours to decrease from full oxygen saturation to hypoxia. Also, low DO is well known to be accompanied by a suite of related changes in sediment and water chemistry, including altered chemical concentrations, changes in nutrient flux, and modified pH (Froelich et al., 1979; Burnett, 1997; Middelburg and Levin, 2009). This is in contrast to the N 2 gas and vacuum degassing methods that strip DO by physically removing it from the water, and thus do not result in the same chemical reactions as SOD. However, using sediments to scrub oxygen more closely resembles how low DO occurs in situ. Bubbling water with N 2 gas to remove oxygen decreases the p CO 2 of the water, and so increases pH, in contrast to oxygen consumption by sediments that typically decreases pH because organic matter remineralization generates CO 2 (Gobler et al., 2014). Though oxygen cannot be drawn down as quickly and the effects of change in DO alone cannot be as cleanly isolated with the SOD method because other chemical characteristics are unavoidably covarying, it more accurately represents DO variability as it would be encountered in natural settings. Also, because this system is closed loop and relies on biological processes to function, issues with excessive buildup of ammonia and nitrates may arise if experiments are run for extended periods without replacing the water in the tanks. This system is best applied in situations that do not require independent control of water chemistry variables and for experiments that can be performed within a short time frame.
MODIFICATIONS AND FUTURE DEVELOPMENT
There are several ways that the user can modify the system to work more effectively or to troubleshoot issues. We divide them into “out-code” modifications, or changes to certain physical or structural features in the system, and “in-code” modifications, or changes to the Arduino code that alter the way the oxygen manipulation is executed.
Out-Code Modifications
The efficacy of the system depends heavily on the oxygen-consuming capacity of the sediments in the experiment tank. Use the most organic-rich sediments available and maximize the ratio of sediment surface area to bulk water volume by using as shallow a tank as possible. Before starting construction of the system, we recommend assessing the oxygen consumption rate the mud can achieve by putting mud into a tank with overlying water to the height anticipated for the experiment, adding a power head to circulate the water, covering the water with bubble wrap, and recording the oxygen through time. If the sediment is not consuming oxygen at a sufficient rate, adding labile organic matter or fertilizer to the sediment or displacing some of the overlying water with a solid object can help increase oxygen drawdown.
Although the power head in the experiment tank may be effective at laterally circulating water, there is still potential for vertical DO gradients to form in the tank, and the steepness of the gradient will increase closer to the sediment surface. Thus, the positioning of the probe in the tank is important. The probe should be secured in position well above the sediment surface and close to the vertical level where study organisms will likely be located. Because the code manipulates DO based on the readings from only one probe, it is also critical to take care in calibrating the sensor, as exemplified by the diel cycle trial ( Figure 3 ). Before it is used, the sensor should be two-point calibrated and its accuracy corroborated at multiple DO concentrations with measurements from a reliable instrument. Though the calibration held well in the diel cycle trial, during longer experiments we advise periodically comparing the DO concentration against a reliable measurement to check for sensor drift.
Because this system was devised for a study designed to observe responses of sediment-dwelling organisms to changing DO, the study organisms were kept in smaller replicate containers filled with sediment within the experiment tank. We constructed a platform that sat on stilts just above the sediment layer to support the replicate containers ( Figure 1 ). This platform had as many gaps as possible so that the water at the sediment surface was well mixed.
In-Code Modifications
Two main features of the code may easily be altered to change the way that DO control is performed: the amount of time the solenoid is held open, and the pause duration between loops.
If DO data are noisy and repeatedly jump substantially above the planned DO concentration, too much oxygenated water may be flowing in with each loop, and the amount of time the solenoid is held open should be decreased. Decreasing the duration between each loop changes the frequency with which the DO is compared to the planned value and manipulated, essentially changing the sensitivity of the system. If this duration is too short, the power head in the tank may not have enough time to circulate the high oxygen water added in the previous loop, resulting in inaccurate measurements and manipulation. The time the solenoid is held open and the pause duration between loops work in concert to affect the precision of DO manipulation, and some amount of trial and error will be necessary to determine the optimal values for each. That said, the system has proven to be somewhat resilient to changes in these variables. We performed a sensitivity test by programming it to maintain DO at 5 mg L –1 for ~1.5 h four times, each with a different combination of the amount of time the valve is left open (either 3 s or 6 s) and the time between loops (either 20 s or 1 min) ( Figure 4 ). In the four trials (3s:20s, 3s:1min, 6s:20s, and 6s:1min), the maximum deviation of the measured DO from the planned DO was 0.21, 0.27, 0.25, and 0.23 mg L –1 , respectively. The percentage of time that the measured value deviated by less than half the maximum deviation was 80%, 91%, 82%, and 81%, respectively. All four trials effectively maintained the programmed DO concentration within a small range of variability.
| Results from sensitivity testing. The system was run four times, programmed to maintain DO at 5 mg L (gray line) for ~1.5 h using different combinations of the amount of time the valve was held open, either 3 s (a and b) or 6 s (c and d), and the time waited between loops, either 20 s (a and c) or 1 min (b and d). |
System Flexibility and Future Development
Aquatic organisms, particularly in coastal areas, exist in an environment with complex variations in DO that have long been difficult to reproduce in a lab setting. Perhaps the most compelling prospect of the described system is its capacity to replicate this variation for study. The programmed oxygen pattern is controlled directly through the Arduino code to allow greater flexibility in the choice, combination, and order of programmed patterns. For example, simply by adjusting the value or equation that the Arduino code is programmed to match and re-uploading the code, the system could be made to alternate increasing and decreasing DO at specific rates, allowing more rigorous study of how the rate of increase or decrease in oxygen affects animal behavior. Or, as was shown in the system test, it can produce a pattern from a modified sine function that mimics a diel oxygen cycle, an extremely common oxygen pattern in shallow coastal waters that remains understudied. The system could further be retrofitted with a high-pressure valve and a small N 2 tank for supplementing with N 2 purging when a more rapid oxygen decrease is needed, or the oxygen probe could be upgraded to an optical sensor for more accurate and precise oxygen manipulation.
The system is also potentially useful for educational applications. It is designed to be as simple and modular as possible, with readily available and reasonably priced components and relatively easy construction. Furthermore, using the code requires some familiarity with the Arduino programming language and can serve as a model of how to use Arduino to build instrumentation for scientific inquiry. The system could be equally employed for classroom behavioral or physiological experiments and as a tool to teach experimental instrument fabrication.
ACKNOWLEDGMENTS
This project was made possible by grants from the gulf of mexico research initiative (gomri) through the alabama center for ecological resilience (acer) consortium and the national science foundation (nsf-oce 1844910 to kmd). kg was supported by a graduate fellowship from the department of marine sciences, university of south alabama. thanks to behzad mortazavi and grant lockridge for helpful discussions..
Gadeken, K.J., and K.M. Dorgan. 2021. A simple and inexpensive method for manipulating dissolved oxygen in the lab. Oceanography 34(2):177–183, https://doi.org/10.5670/oceanog.2021.202 .
- Bevan, D.J., and D.L. Kramer. 1988. A control system for the long-term maintenance of hypoxic water. Aquaculture 71(1–2):165–169, https://doi.org/10.1016/0044-8486(88)90283-9 .
- Burnett, L.E. 1997. The challenges of living in hypoxic and hypercapnic aquatic environments. American Zoologist 37(6):633–640, https://doi.org/10.1093/icb/37.6.633 .
- Calder-Potts, R., J.I. Spicer, P. Calosi, H.S. Findlay, and S. Widdicombe. 2015. A mesocosm study investigating the effects of hypoxia and population density on respiration and reproductive biology in the brittlestar Amphiura filiformis . Marine Ecology Progress Series 534:135–147, https://doi.org/10.3354/meps11379 .
- Diaz, R.J., and R. Rosenberg. 1995. Marine benthic hypoxia: A review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanography and Marine Biology: An Annual Review 33:245–303.
- Diaz, R.J., and R. Rosenberg. 2011. Introduction to environmental and economic consequences of hypoxia. International Journal of Water Resources Development 27(1):71–82, https://doi.org/10.1080/07900627.2010.531379 .
- Foster, S.Q., and R.W. Fulweiler. 2019. Estuarine sediments exhibit dynamic and variable biogeochemical responses to hypoxia. Journal of Geophysical Research: Biogeosciences 124(4):737–758, https://doi.org/10.1029/2018JG004663 .
- Froelich, P., G.P. Klinkhammer, M.L. Bender, N.A. Luedtke, G.R. Heath, D. Cullen, P. Dauphin, D. Hammond, B. Hartman, and V. Maynard. 1979. Early oxidation of organic matter in pelagic sediments of the eastern equatorial Atlantic: Suboxic diagenesis. Geochimica et Cosmochimica Acta 43(7):1,075–1,090, https://doi.org/10.1016/0016-7037(79)90095-4 .
- Gobler, C.J., E.L. DePasquale, A.W. Griffith, and H. Baumann. 2014. Hypoxia and acidification have additive and synergistic negative effects on the growth, survival, and metamorphosis of early life stage bivalves. PLoS ONE 9(1):e83648, https://doi.org/10.1371/journal.pone.0083648 .
- Grecay, P.A., and K.L. Stierhoff. 2002. A device for simultaneously controlling multiple treatment levels of dissolved oxygen in laboratory experiments. Journal of Experimental Marine Biology and Ecology 280(1–2):53–62, https://doi.org/10.1016/S0022-0981(02)00379-9 .
- Lehrter, J.C., D.L. Beddick, R. Devereux, D.F. Yates, and M.C. Murrell. 2012. Sediment-water fluxes of dissolved inorganic carbon, O 2 , nutrients, and N 2 from the hypoxic region of the Louisiana continental shelf. Biogeochemistry 109(1–3):233–252, https://doi.org/10.1007/s10533-011-9623-x .
- Levin, L.A., W. Ekau, A.J. Gooday, F. Jorissen, J.J. Middelburg, S.W.A. Naqvi, C. Neira, N.N. Rabalais, and J. Zhang. 2009. Effects of natural and human-induced hypoxia on coastal benthos. Biogeosciences (6):2,063–2,098, https://doi.org/10.5194/bg-6-2063-2009 .
- Long, W.C., B.J. Brylawski, and R.D. Seitz. 2008. Behavioral effects of low dissolved oxygen on the bivalve Macoma balthica . Journal of Experimental Marine Biology and Ecology 359(1):34–39, https://doi.org/10.1016/j.jembe.2008.02.013 .
- McCarthy, M.J., K.S. McNeal, J.W. Morse, and W.S. Gardner. 2008. Bottom-water hypoxia effects on sediment–water interface nitrogen transformations in a seasonally hypoxic, shallow bay (Corpus Christi Bay, TX, USA). Estuaries and Coasts 31(3):521–531, https://doi.org/10.1007/s12237-008-9041-z .
- Middelburg, J.J., and L.A. Levin. 2009. Coastal hypoxia and sediment biogeochemistry. Biogeosciences 6(7):1,273–1,293, https://doi.org/10.5194/bg-6-1273-2009 .
- Miller, D.C., D.E. Body, J.C. Sinnett, S.L. Poucher, J. Sewall, and D.J. Sleczkowski. 1994. A reduced dissolved oxygen test system for marine organisms. Aquaculture 123(1–2):167–171, https://doi.org/10.1016/0044-8486(94)90129-5 .
- Mount, D.I. 1961. Development of a system for controlling dissolved-oxygen content of water. Transactions of the American Fisheries Society 90(3):323–327, https://doi.org/10.1577/1548-8659(1961)90[323:DOASFC]2.0.CO;2 .
- Neubacher, E.C., R.E. Parker, and M. Trimmer. 2013. The potential effect of sustained hypoxia on nitrogen cycling in sediment from the southern North Sea: A mesocosm experiment. Biogeochemistry 113(1–3):69–84, https://doi.org/10.1007/s10533-012-9749-5 .
- Peterson, M.S., and B.E. Ardahl. 1992. Automated maintenance of dissolved oxygen concentrations in flow-through aquaria. Aquaculture 101(3–4):379–384, https://doi.org/10.1016/0044-8486(92)90040-R .
- Riedel, B., T. Pados, K. Pretterebner, L. Schiemer, A. Steckbauer, A. Haselmair, M. Zuschin, and M. Stachowitsch. 2014. Effect of hypoxia and anoxia on invertebrate behaviour: Ecological perspectives from species to community level. Biogeosciences 11(6):1,491–1,518, https://doi.org/10.5194/bg-11-1491-2014 .
- Sturdivant, S.K., R.J. Díaz, and G.R. Cutter. 2012. Bioturbation in a declining oxygen environment, in situ observations from Wormcam. PLoS ONE 7(4):e34539, https://doi.org/10.1371/journal.pone.0034539 .
- Thetmeyer, H., U. Waller, K.D. Black, S. Inselmann, and H. Rosenthal. 1999. Growth of European sea bass ( Dicentrarchus labrax L.) under hypoxic and oscillating oxygen conditions. Aquaculture 174(3–4):355–367, https://doi.org/10.1016/S0044-8486(99)00028-9 .
- Wenner, E., D. Sanger, M. Arendt, A.F. Holland, and Y. Chen. 2004. Variability in dissolved oxygen and other water-quality variables within the national estuarine research reserve system. Journal of Coastal Research (45):17–38, https://doi.org/10.2112/SI45-017.1 .
Copyright & Usage
This is an open access article made available under the terms of the Creative Commons Attribution 4.0 International License ( https://creativecommons.org/licenses/by/4.0/ ), which permits use, sharing, adaptation, distribution, and reproduction in any medium or format as long as users cite the materials appropriately, provide a link to the Creative Commons license, and indicate the changes that were made to the original content. Images, animations, videos, or other third-party material used in articles are included in the Creative Commons license unless indicated otherwise in a credit line to the material. If the material is not included in the article’s Creative Commons license, users will need to obtain permission directly from the license holder to reproduce the material.
Remember Me

Shop Experiment Dissolved Oxygen in Water Experiments
Dissolved oxygen in water.
Experiment #12A from Advanced Biology with Vernier
Introduction
Although water is composed of oxygen and hydrogen atoms, biological life in water depends upon another form of oxygen—molecular oxygen. Oxygen is used by organisms in aerobic respiration, where energy is released by the combustion of sugar in the mitochondria. This form of oxygen can fit into the spaces between water molecules and is available to aquatic organisms.
Fish, invertebrates, and other aquatic animals depend upon the oxygen dissolved in water. Without this oxygen, they would suffocate. Some organisms, such as salmon, mayflies, and trout, require high concentrations of oxygen in their water. Other organisms, such as catfish, midge fly larvae, and carp can survive with much less oxygen. The ecological quality of the water depends largely upon the amount of oxygen the water can hold. The quality of the water can be assessed with fair accuracy by observing the aquatic animal populations in a stream.
In this experiment, you will
- Use a Dissolved Oxygen Probe to measure the concentration of dissolved oxygen in water.
- Study the effect of temperature on the amount of dissolved oxygen in water.
- Predict the effect of water temperature on aquatic life.
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.

Ready to Experiment?
Ask an expert.
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email [email protected]
Purchase the Lab Book
This experiment is #12A of Advanced Biology with Vernier . The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.


Teacher Resource Center
Pasco partnerships.

2024 Catalogs & Brochures
What factors affect dissolved oxygen.
Students will use a dissolved oxygen sensor to determine how different factors affect the concentration of dissolved oxygen in water.
Grade Level: High School
Subject: Biology • Environmental Science
Student Files
| 123.42 KB | ||
| 125.54 KB |
Teacher Files
Sign In to your PASCO account to access teacher files and sample data.
Featured Equipment

Wireless Optical Dissolved Oxygen Sensor
This sensor is perfect for the live or long-term monitoring of dissolved oxygen concentration and saturation, in both the lab and field.
Many lab activities can be conducted with our Wireless , PASPORT , or even ScienceWorkshop sensors and equipment. For assistance with substituting compatible instruments, contact PASCO Technical Support . We're here to help. Copyright © 2019 PASCO
More Experiments
High School
- Regulation of Body Heat
- Spirometry: Pulmonary Function
- Respiration of Germinating Seeds (PASPORT sensors)
- Volume of Breath (PASPORT sensors)
- Enzyme Action (Optical Dissolved Oxygen)
Middle School
- Program a Greenhouse Sense and Control System (Advanced)

IMAGES
VIDEO
COMMENTS
Laboratory Experiment 6: Dissolved Oxygen (DO) Objective: Determine DO content of a given sample Background: Dissolved oxygen (DO) levels in environmental water depend on the physiochemical and biochemical activities in water body and it is an important useful in pollution and waste treatment process control. ...
The dissolved oxygen concentration of seawater is defined as the number of milliliters of dioxygen gas (O2 ) per liter of seawater (mL L -1 ). 4. Principle of Analysis. The chemical determination of oxygen concentrations in seawater is based on the method first proposed by Winkler (1888) and modified by Strickland and Parsons (1968).
The Winkler Method is a technique used to measure dissolved oxygen in freshwater systems. Dissolved oxygen is used as an indicator of the health of a water body, where higher dissolved oxygen concentrations are correlated with high productivity and little pollution. This test is performed on-site, as delays between sample collection and testing ...
The Winkler method for dissolved oxygen (DO) is a standard titration technique to measure the oxygen content in water. Water is collected in a sample bottle with no air that could interfere with the DO reading. Fixed reagents are added to the sample to form an acidic compound. The sample is then neutralized using a neutralizing compound to ...
1.1 Dissolved Oxygen (DO) Standardization of Na 2 S2 O 3 Solution Potassium bi-iodate (KH(IO 3)2) is first reduced to an equivalent amount of iodine by the addition of KI. This iodine is then quantified by titration with Na 2 S2 O 3 as described in experiment 5A. Note: Write the relevant equations for the above reactions. Procedure:
Dear viewer/subscriber, if my videos helped you a lot (maybe you aced your exams as a student, or you won the admiration and full attention of your students ...
Dissolved oxygen (DO) is a measure of how much oxygen is dissolved in a sample. The amount of dissolved oxygen in water can tell us about the water quality. ...
Saturation of 91 to 110% dissolved oxygen is considered excellent; between 71 and 90% is good, 51-70% is fair, and below 50% is poor. Dissolved oxygen levels of 6 mg/L are sufficient to support most aquatic species. Levels below 4 mg/L are stressful to the majority of aquatic animals, so biodiversity will be affected.
Introduction. Dissolved oxygen is one of the primary indicators of the quality of an aquatic environment. Oxygen enters water from the surrounding air, as a product of photosynthesis, and as a result of rapid movement of water. A Dissolved Oxygen Probe can be used in a wide variety of tests or investigations to determine dissolved oxygen ...
Experiment 5: Dissolved Oxygen (4500-O. C. Azide Modification) OBJECTIVES: To determine Dissolved Oxygen (D.O) concentration in a water sample. BACKGROUND AND PRINCIPLE: The test was first developed by Lajos Winkler while working on his doctoral dissertation in 1888. Dissolved oxygen (D.O) levels in environmental water depend on the physiochemical
Dissolved oxygen levels can be measured by a basic chemical analysis method (titration method), an electrochemical analysis method (diaphragm electrode method), and a photochemical analysis method (fluorescence method). The diaphragm electrode method is the most widely used method. Titration Method. Diaphragm Electrode Method.
06) Aquatic Photosynthesis and Dissolved Gases. Students use a Wireless Optical Dissolved Oxygen sensor (PS-3246), Wireless CO₂ sensor (PS-3208), Dissolved CO₂ Waterproof Sleeve (PS-3545), and a Photosynthesis Chamber (PS-3251) to compare the metabolic products of Elodea in light and dark conditions.
Testing dissolved oxygen (DO) in water is either measured via chemical analysis such as a titrimetric method, electroanalytical (using galvanic & ... Mini Lab Grade Dissolved Oxygen Probe $ 134.99 USD. Add to cart. Lab Grade Dissolved Oxygen Probe $ 259.99 - $ 261.99 USD. Select options. Industrial Dissolved Oxygen Probe $ 349.99 USD.
Dissolved Oxygen and Temperature Lab Background: In this experiment, you will try to find out what happens to the amount of dissolved oxygen when the temperature of the water increases or decreases. This will help you understand how seasonal changes as well as long-term changes, such as climate change, might affect aquatic ecosystems.
Field and lab meters to measure dissolved oxygen have been around for a long time. As this picture shows, modern meters are small and highly electronic. They still use a probe, which is located at the end of the cable. Dissolved oxygen is dependent on temperature (an inverse relation), so the meter must be calibrated properly before each use.
1.0. Oxygen gas is dissolved in water by a variety of processes—diffusion between the atmosphere and water at its surface, aeration as water flows over rocks and other debris, churning of water by waves and wind, and photosynthesis of aquatic plants. There are many factors that affect the concentration of dissolved oxygen in an aquatic ...
Many lab activities can be conducted with our Wireless, PASPORT, or even ScienceWorkshop sensors and equipment. For assistance with substituting compatible instruments, contact PASCO Technical Support. We're here to help. Students will use a dissolved oxygen sensor to determine how different factors affect the concentration of dissolved oxygen ...
Laboratory Testing of Dissolved Oxygen. If you use a meter and probe, you must do the testing in the field; dissolved oxygen levels in a sample bottle change quickly due to the decomposition of organic material by microorganisms or the production of oxygen by algae and other plants in the sample. This will lower your DO reading.
ASSESSMENT. To serve as a usable method for manipulating oxygen in the lab, the system must reliably, precisely, and accurately produce the programmed DO patterns in the experiment tank. We tested the system by programming it to generate a diel oxygen cycle, with DO concentrations ranging from 3 mg L -1 to 7 mg L -1.
Oxygen is used by organisms in aerobic respiration, where energy is released by the combustion of sugar in the mitochondria. This form of oxygen can fit into the spaces between water molecules and is available to aquatic organisms. Fish, invertebrates, and other aquatic animals depend upon the oxygen dissolved in water.
Dissolved Oxygen and Temperature Lab Background: In this experiment, you will try to find out what happens to the amount of dissolved oxygen when the temperature of the water increases or decreases. This will help you understand how seasonal changes as well as long-term changes, such as climate change, might affect aquatic ecosystems.
Many lab activities can be conducted with our Wireless, PASPORT, or even ScienceWorkshop sensors and equipment. For assistance with substituting compatible instruments, contact PASCO Technical Support. We're here to help. Students will use a dissolved oxygen sensor to determine how different factors affect the concentration of dissolved oxygen ...