
Study of Osmosis by Potato Osmometer
A study of osmosis can be done using a potato osmometer. Osmosis is a phenomenon in which water moves from high solvent to low solvent concentration. The movement of water occurs between two compartments, separated by a semipermeable membrane .
The cell membrane of living organisms behaves as a semipermeable or selective membrane. The permeability of a selective membrane differs based on the size, charge and mass of different molecules.
Biological membranes are impermeable to large biomolecules and polar molecules like ions. But, non-polar molecules (lipids) and small molecules (oxygen, carbon dioxide etc.) can cross the selective barrier.
Water is the solvent that travels down or up the cell concentration gradient through osmosis. We can study water diffusion by creating two compartments and a semipermeable membrane in between.
The difference in the concentration of solutes or solvents between two compartments is the driving force responsible for water movement. Here, we need to note that only solvents can pass the selective barrier, not solutes.
Thus, the diffusion or distribution of water is related to osmosis . This post describes the meaning, requirements, procedure and results of the potato osmometer experiment.
Content: Study of Osmosis by Potato Osmometer
Potato osmometer, materials required, precautions.
It is a common experiment to demonstrate both endosmosis and exosmosis using a potato. Using a potato Osmoscope, we can study osmosis in a living system.
Here, a potato is used because the porous outer surface of the potato acts as a selective membrane .
- The contents within the cell form one compartment.
- The solution surrounding the cell forms another compartment.
Thus, a selective membrane separates two compartments and allows the process of osmosis .
- High solvent concentration in the cell surrounding.
- Low solvent concentration in the cavity of potato tuber.
Following the rule of osmosis, water in the cell surrounding enters the tuber cavity via the cell membrane.
- High solvent concentration in the cavity of potato tuber.
- Low solvent concentration in the cell surrounding.
Following the rule of osmosis, water in the potato cavity enters the surrounding solution via the cell membrane.
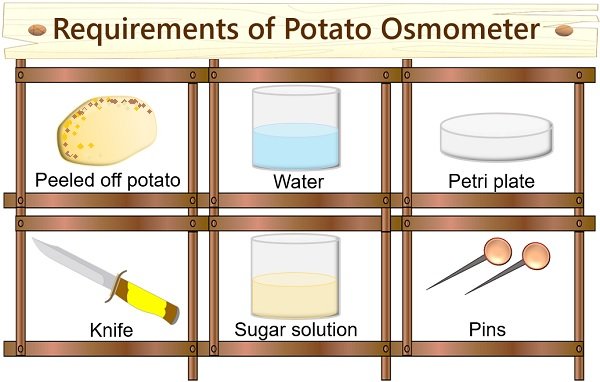
- Peeled off potato
- Concentrated sugar solution
- Petri plate
Video: Study of Osmosis
To perform the potato osmometer experiment, we need to follow the given procedure:
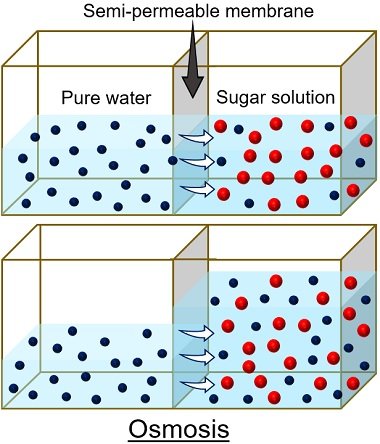
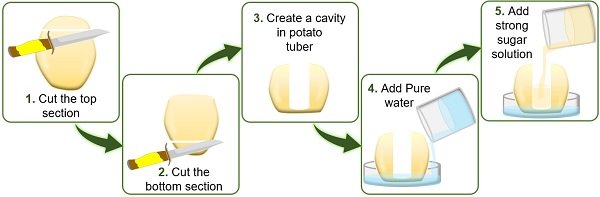
- First, peel off the large-sized potato using a peeler or knife.
- Then cut the upper and lower portions of the peeled potato using a knife. Through this step, we can easily place the potato on the Petri plate.
- Using a knife, make a cavity from the centre of the potato deep into the bottom, leaving some space. Here, the bottom of the potato will function as a selective membrane.
- Then, keep the potato on the Petri plate.
- To study endosmosis , pour water into half of the Petri plate. Next, pour the concentrated sugar solution into half of the cavity created in the potato.
- To study exosmosis , add concentrated sugar solution on the Petri plate and water into the cavity of the potato tuber.
- Then, fix a pin into the potato tuber-A and B to mark the level of sugar solution and water added into the cavity.
- Leave the plate undisturbed for some time until you notice any change.
Observation
- Observe the level of sugar solution in the cavity of potato tuber-A.
- Notice the level of water in the cavity of the potato tuber-B.
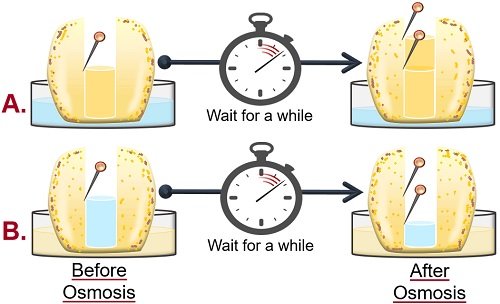
Potato Osmosis Experiment Results
- The level of sugar solution in the cavity of potato tuber-A increases . It occurs because the water in the Petri plate will move towards the cell with a high solute or low solvent concentration. This experiment shows endosmosis , as water goes into the cell or potato tuber.
- In contrast, the level of water in the cavity of potato tuber-B decreases . Here, water in the cavity moves toward the solution in the Petri plate due to the high solute concentration in the surrounding. This experiment shows exosmosis as water leaves the cell or potato tuber.
- The cavity should be deep enough by leaving a minimum thickness at the bottom.
- The sugar solution should have a high osmotic concentration.
The water movement from the Petri plate to the potato cavity or vice versa is due to the difference in the solvent or solute concentration between the two compartments.
Related Topics:
- Germination of Plant
- Difference Between Root and Stem
- Nerve Impulse
- Ozone Formation
- Examples of Adsorption in Daily Life
Leave a Comment Cancel Reply
Your email address will not be published. Required fields are marked *
Start typing and press enter to search
- Biology Article
- Study Of Osmosis By Potato Osmometer
Understanding Osmosis Using Potato Osmometer
To study by demonstrating the osmosis process by potato osmometer.
What is Osmosis?
Osmosis is the phenomena in which solvent molecules pass through a semi-permeable membrane from an area of higher concentration to an area of lower concentration. The process continues until the quantity of fluid is balanced or equalized in both regions, the region of higher concentration and the region of lower concentration of the semipermeable membrane. In other words, osmosis is the diffusion or movement of water from a region of higher water potential to a region of lower water potential.
In osmosis, what are solvent and solute?
The fluid that permeates through the semipermeable membrane is called the solvent, whereas the solute is the dissolved particles in the fluid.
What is the solution?
The mixture of solute and solvent form the solution.
List the different types of solutions.
The following are the types of solutions:
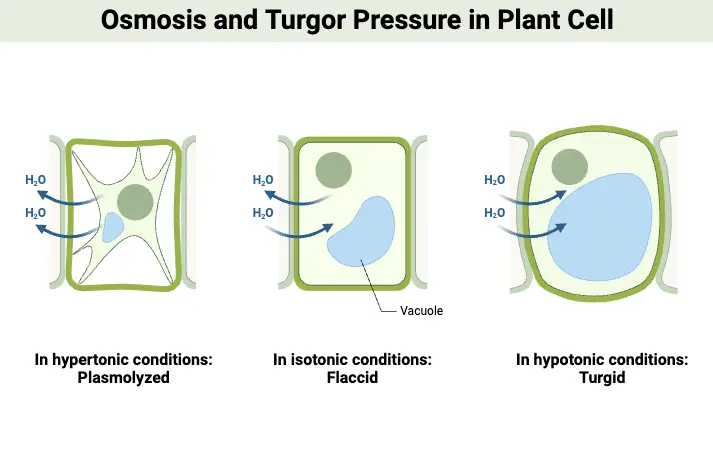
- Hypertonic solution – It is a solution with a high solute level. If living cells are placed in a hypertonic solution, because of lower concentration water moves out of the cell causing it to shrink and becomes plasmolyzed.
- Hypotonic solution – It is a solution with low concentration levels of solute. If living cells are placed in this solution, water passes into the cells because of higher water concentration in comparison to the cell causing the cells to swell and turn turgid.
- Isotonic solution – A solution is said to be isotonic if both solutions have an equal concentration of solute. If living cells are placed in an isotonic solution, no change is shown as there is the equal concentration on both the regions hence the cell retains its original shape.
Material Required
- A fresh large-sized potato tuber
- 20% sucrose solution
- Scalpel/blade
- A Bell pin needle that is labelled with a waterproof ink
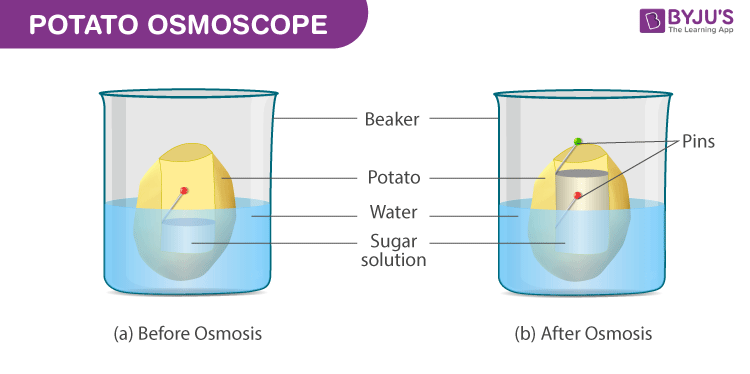
- Slice the potato tuber into two equal halves with the help of a scalpel or a blade. The outer skin is to be peeled off. Since the tuber shape is irregular, slice the halves into squares
- From the mid-region of the tuber, scoop from the soft parenchyma, so as to form a tiny cavity of a square or a circular shape. At the base, the cavity prepared should have a minimum thickness.
- Fill up half the cavity with the freshly prepared 20% sugar solution. Into the cavity, fix a pin in a way that the mark is in the same line with the layer of the sucrose solution.
- Set up the osmometer in a Petri dish/beaker that is filled with water in a way such that 75% of the potato osmometer is immersed in water
- The set up should remain uninterrupted for close to 1 hour.
- Notice the sugar solution in the osmometer towards the end of the experiment
- Carry out the experiment with the help of water in the cavity and the sucrose solution in the petri dish/beaker.
Observation
After a period of time, within the osmoscope, the sugar solution rises and is seen coloured.
- An increase in the level of sucrose solution is observed in the osmometer. It is because of the entrance of water due to endosmosis from the beaker.
- Also, a water potential gradient is built between the sucrose solution in the external water and the osmometer.
- Though both the liquids are divided by living cells of the potato tuber, they allow the entrance of water into the sugar solution.
- This demonstrates the entrance of water into the sugar solution through the tissues of potato serving as a selectively permeable membrane.
Viva Questions
Q.1. What is plasmolysis?
A.1. It is a process, wherein the protoplasm of the plant cell turns round as a result of contraction when placed in a hypertonic solution due to exosmosis resulting in the decline in the tension of the cell wall.
Q.2. What is the significance of osmosis?
A.2. Osmosis maintains cell turgidity. It causes the transportation of nutrients and discharge of metabolic waste products. It preserves the interior environment of a living entity to maintain an equilibrium between the intracellular fluid levels and water.
Q.3. What is diffusion?
A.3. The movement of molecules from a region of higher concentration to a region of lower concentration. Osmosis is a type of diffusion.
For more information on related biological concepts and experiments, please register at BYJU’S.
Related Links:

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Biology related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
Good Thank you BYJU’S!!
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.
Osmosis – Definition, Types, Mechanism, Significance, Examples
- AP Biology Note Podcast Free
- DeepDive into Cell Biology Podcast Free
- DeepDive into Biochemistry Podcast Free
What is Osmosis?
Definition of osmosis, how does osmosis work, factors affecting osmosis, types of osmosis, what is osmotic solution (tonicity), variation of osmosis, what is osmotic pressure, significance of osmosis, examples of osmosis, difference between osmosis and diffusion, study of osmosis by potato osmometer.
- Osmosis is a fundamental biological process defined as the spontaneous movement of solvent molecules through a selectively permeable membrane. This movement occurs from a region of high water potential, characterized by lower solute concentration, to a region of low water potential, where solute concentration is higher. The ultimate goal of osmosis is to equalize solute concentrations on both sides of the membrane, facilitating equilibrium. This phenomenon is essential for maintaining homeostasis in biological systems.
- In more technical terms, osmosis refers specifically to the movement of water across membranes that permit the passage of water but restrict the movement of solutes. The concept of osmotic pressure is crucial here; it is the external pressure needed to halt the net movement of solvent molecules across the membrane. This pressure is a colligative property, which means it is determined by the solute’s molar concentration rather than its specific identity. Thus, various solutes can exert different osmotic pressures depending on their concentrations in a solution.
- Biological membranes are inherently semipermeable, meaning they allow certain molecules to pass while blocking others. Generally, these membranes prevent the passage of large and polar molecules, such as ions and proteins , while permitting non-polar or hydrophobic molecules, as well as small gases like oxygen and carbon dioxide, to pass. The permeability of a membrane is influenced by several factors, including solute size, charge, and solubility.
- Water plays a crucial role in osmosis, as it is the most common solvent in biological systems. Water molecules travel through the plasma membrane , tonoplast membrane, or organelle membranes by diffusing across the phospholipid bilayer. This process is often facilitated by specialized proteins called aquaporins, which function similarly to ion channels, allowing for efficient water transport .
- Osmosis serves several vital functions in biological contexts. For instance, it is the primary mechanism by which cells regulate water content. When a cell is placed in a hypotonic solution (one with lower solute concentration), water flows into the cell, leading to increased turgor pressure . Conversely, in a hypertonic solution (higher solute concentration), water exits the cell, resulting in a decrease in turgor pressure and potential cell shrinkage.
- A practical demonstration of osmosis can be observed with potato slices placed in saline solutions. When these potato slices are submerged in a concentrated salt solution, water moves out of the cells into the surrounding solution, causing the potato to lose turgor pressure and shrink. The degree of shrinkage correlates with the concentration of the salt solution: the higher the concentration, the greater the loss of water and subsequent reduction in size.
- The history of osmosis can be traced back to ancient observations, with significant advancements made over time. The first formal documentation of osmosis occurred in 1748 by Jean-Antoine Nollet. The term “osmosis” itself is derived from the Greek words for “push” and “within,” reflecting the movement of water molecules driven by concentration gradients. In 1867, Moritz Traube’s invention of selective precipitation membranes significantly improved the understanding and measurement of osmotic flow, further solidifying the importance of this phenomenon in both biological and chemical contexts.
Osmosis is the spontaneous movement of solvent molecules, typically water, through a selectively permeable membrane from a region of lower solute concentration to a region of higher solute concentration, aiming to equalize solute concentrations on both sides.
Osmosis is a fundamental process that facilitates the movement of water across a selectively permeable membrane. It occurs when two solutions with differing solute concentrations are separated by this membrane. Understanding the mechanisms behind osmosis involves exploring the forces that drive water movement and the structural components involved in the process.
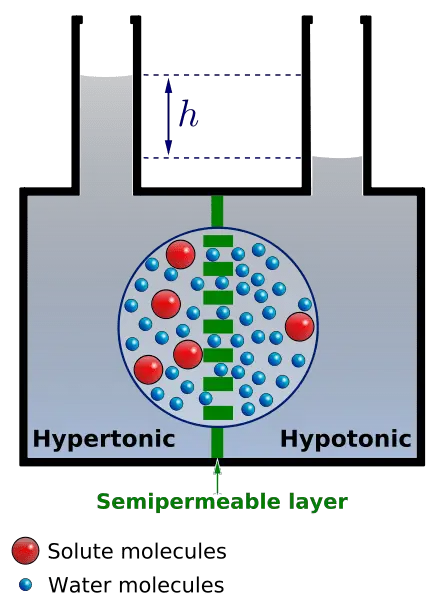
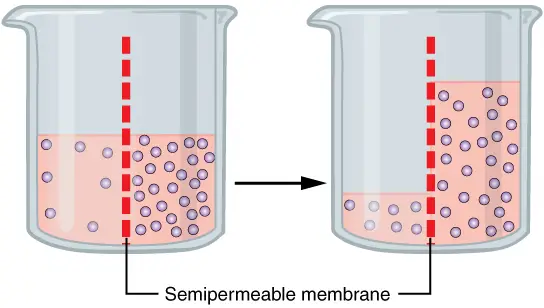
- Movement Across Membranes : In osmosis, water molecules move from a region of high water concentration (low solute concentration) to a region of low water concentration (high solute concentration). This movement continues until an equilibrium is reached, where there is no net flow of water.
- Role of Semipermeable Membranes : A critical requirement for osmosis is the presence of a semipermeable membrane. Such membranes allow water molecules to pass while restricting the movement of solute molecules. Without this membrane, the process resembles diffusion rather than osmosis.
- Concentration Gradient : The driving force behind osmosis is the concentration gradient of water across the membrane. Water moves downhill, meaning it seeks to equalize the concentration differences by flowing toward areas with higher solute concentrations.
- Chemical Potential : The concept of chemical potential is essential to understanding osmosis. The chemical potential of pure water differs from that of water in a solution containing solute molecules. The presence of solute reduces the pressure exerted by water molecules, creating a difference in potential.
- Pressure and Equilibrium : As water molecules move from the pure water side to the solute side, they exert pressure that continues until the chemical potential equilibrates. This pressure results from the water molecules in the pure solution pushing toward the area with a higher solute concentration.
- Osmotic Pressure Gradient : Osmosis is driven by the osmotic pressure gradient, which is the difference in osmotic pressures between the two solutions. This gradient dictates the tendency of water molecules to migrate from one area to another.
- Water Potential : The relative tendency of water to move is often quantified by water potential, denoted by the Greek letter Ψ (Psi). Water potential is a measure that encompasses both solute potential and pressure potential, influencing the direction and extent of water movement.
- Channel Proteins : Water molecules, being polar, require assistance to traverse cell membranes effectively. Channel proteins embedded within the membrane provide hydrophilic pathways, facilitating the movement of water along the concentration gradient. These proteins are vital for the efficient transport of water, ensuring that osmosis can occur swiftly and effectively.
Osmosis, the movement of solvent molecules across a selectively permeable membrane, is influenced by several critical factors. Understanding these factors helps elucidate how osmosis operates in various biological and chemical contexts.
- Temperature : The rate of osmosis is directly influenced by temperature. As the temperature of a system increases, the kinetic energy of the molecules also rises. This heightened energy results in increased molecular movement, thereby accelerating the process of osmosis. Consequently, higher temperatures facilitate faster rates of water movement across the membrane.
- Concentration Gradient : The concentration gradient is a pivotal driving force in osmosis. When there is a significant difference in solute concentration across the membrane, osmosis is expedited. In a scenario where one solution contains a higher concentration of solute than another, the pressure exerted by the solvent molecules decreases in the more concentrated solution. This decline in pressure enhances the movement of solvent molecules toward the area of higher solute concentration. However, once equilibrium is achieved and solute concentrations equalize, the net movement of water ceases.
- Water Potential/Solvent Potential : The water potential across a semi-permeable membrane is another determinant of the osmosis rate. Solutions with higher water potential allow for increased movement of water molecules across the membrane, as the pressure exerted by the particles within the solution is greater. As the osmotic movement continues, water potential will eventually equilibrate on both sides of the membrane. Once equilibrium is established, water molecules continue to flow, but in equal amounts, maintaining a stable balance between the solutions.
- Surface Area and Membrane Thickness : The characteristics of the membrane also significantly impact the rate of osmosis. An increase in surface area enhances the available space for molecular movement, thus facilitating a higher rate of osmosis. Conversely, a reduction in surface area limits molecular movement and restricts the process. Furthermore, the thickness of the membrane plays a crucial role; as membrane thickness increases, the rate of osmosis tends to decrease due to the longer diffusion pathway for water molecules.
- Pressure : Pressure is a fundamental factor affecting osmosis. When external pressure is applied to the system, it can influence the direction of osmotic movement. If the pressure exceeds the natural pressure exerted by the solvent molecules, the direction of osmosis may reverse, causing solvent molecules to migrate toward the region with higher solvent concentration. Conversely, if the applied pressure is less than that exerted by the solvent molecules, it does not change the direction but can reduce the overall rate of osmosis. When pressure is applied in the same direction as the concentration gradient, it can enhance the rate of osmosis by facilitating increased movement of solvent molecules.
Osmosis can be categorized into two distinct types based on the direction of solvent movement relative to the cell: endosmosis and exosmosis. Each type of osmosis plays a crucial role in maintaining cellular homeostasis and influencing cell behavior in different environments.
- Endosmosis : This type of osmosis occurs when a cell is placed in a hypotonic solution, which has a lower solute concentration compared to the cytoplasm of the cell. In this scenario, solvent molecules, typically water, move into the cell. As water enters, the cell absorbs this influx and swells, becoming turgid. This phenomenon is crucial for plant cells, as turgidity helps maintain structural integrity and support. The process of endosmosis can also be described as deplasmolysis, where the cell regains water after having previously lost it, thus restoring its normal shape and functionality.
- Exosmosis : Conversely, exosmosis takes place when a cell is immersed in a hypertonic solution, characterized by a higher solute concentration relative to the cell’s interior. In this situation, solvent molecules exit the cell, leading to a loss of water. As a result, the cell becomes flaccid, undergoing plasmolysis , which can impair its functions and lead to cell shrinkage. Exosmosis is significant in various physiological processes, such as the regulation of water balance in cells and tissues .
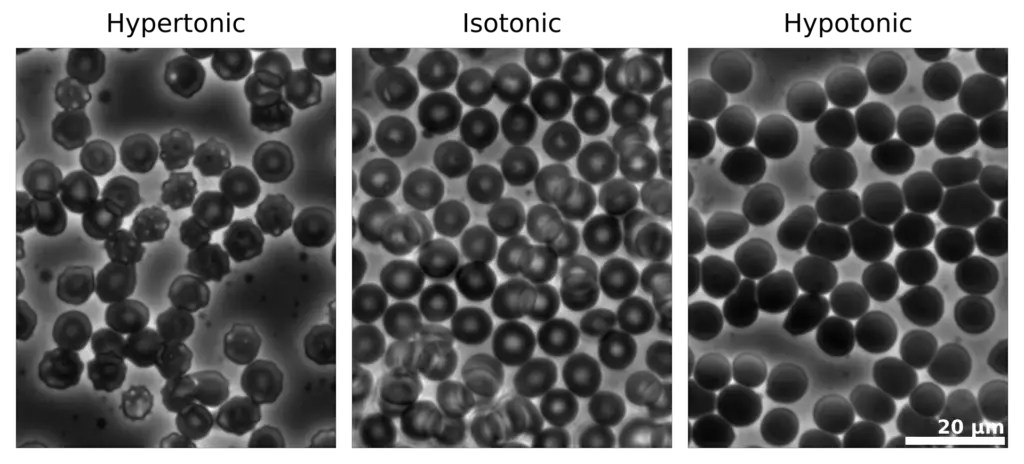
Tonicity refers to the ability of extracellular solutions to influence the movement of water across cell membranes through the process of osmosis. It is fundamentally determined by the concentration of solute and solvent molecules in a solution, which dictates how water will flow in relation to the cell’s internal environment. Solutions can be classified into three categories based on their tonicity: hypotonic, hypertonic, and isotonic.

- Hypotonic Solution : A solution is considered hypotonic when it contains a lower concentration of solute compared to the interior of a cell. In this context, water moves into the cell, leading to endosmosis. As a result, the cell may swell and potentially burst due to the increased internal pressure. This phenomenon is crucial for plant cells, which rely on turgor pressure for structural integrity.
- Hypertonic Solution : Conversely, a hypertonic solution has a higher concentration of solute compared to the cell’s interior. When a cell is placed in a hypertonic solution, water exits the cell, causing exosmosis. This movement results in cell shrinkage, impairing its ability to function and divide. This process is significant in various physiological contexts, including cellular dehydration.
- Isotonic Solution : An isotonic solution has an equal concentration of solute as that found within the cell. In such scenarios, there is no net movement of water across the cell membrane , leading to a stable cell size. This balance is essential for maintaining homeostasis within the cell, as it ensures that cellular functions can proceed without disruption from osmotic stress.
Osmosis is a vital process that varies in its mechanisms and applications, particularly in its forward and reverse forms. These variations highlight the versatility of osmotic principles in different contexts, such as water purification and nutrient separation.
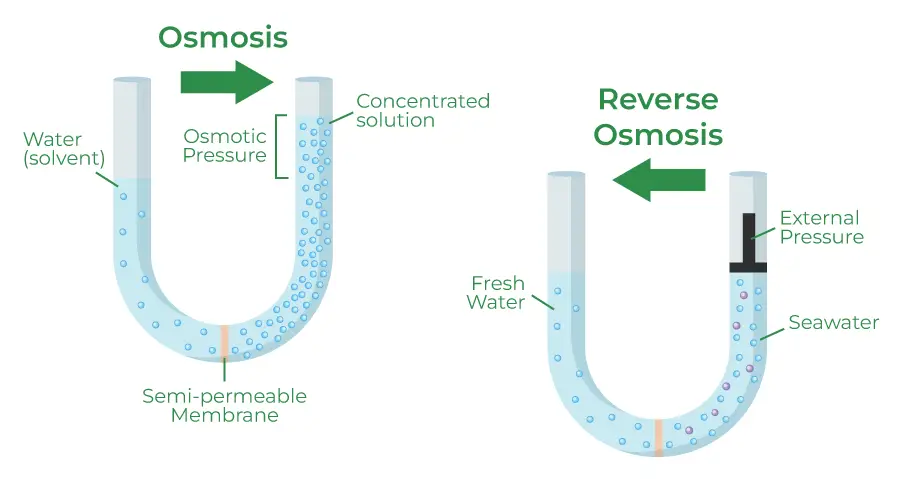
- Reverse osmosis involves applying pressure to drive a solvent through a semipermeable membrane, effectively filtering out solutes. In this process, pure solvent is forced from an area of higher solute concentration to an area of lower solute concentration.
- The pressure applied must exceed the osmotic pressure of the solution, allowing the selective separation of water while retaining dissolved substances on one side of the membrane. This method is widely utilized for water purification and desalination, enabling the production of potable water from saline or contaminated sources.
- Forward osmosis operates on the principle of using a draw solution with higher osmotic pressure than the feed solution to separate water from a mixture containing undesirable solutes. In this variation, water moves through a semipermeable membrane towards the draw solution, resulting in the dilution of the draw solution and the concentration of the feed solution.
- The movement of water induced by the osmotic pressure gradient leads to a net flow that can be advantageous in various applications. The diluted draw solution can either be used directly or undergo a secondary separation process to extract the draw solute, which can often be more efficient compared to reverse osmosis alone.
Osmotic pressure is a fundamental concept in biology and chemistry, representing the pressure required to prevent the flow of solvent molecules across a semi-permeable membrane. This pressure is crucial for regulating the movement of water in biological systems, influencing cell hydration and homeostasis. It serves as the driving force behind osmosis, where solvent molecules move from areas of lower solute concentration to areas of higher solute concentration.
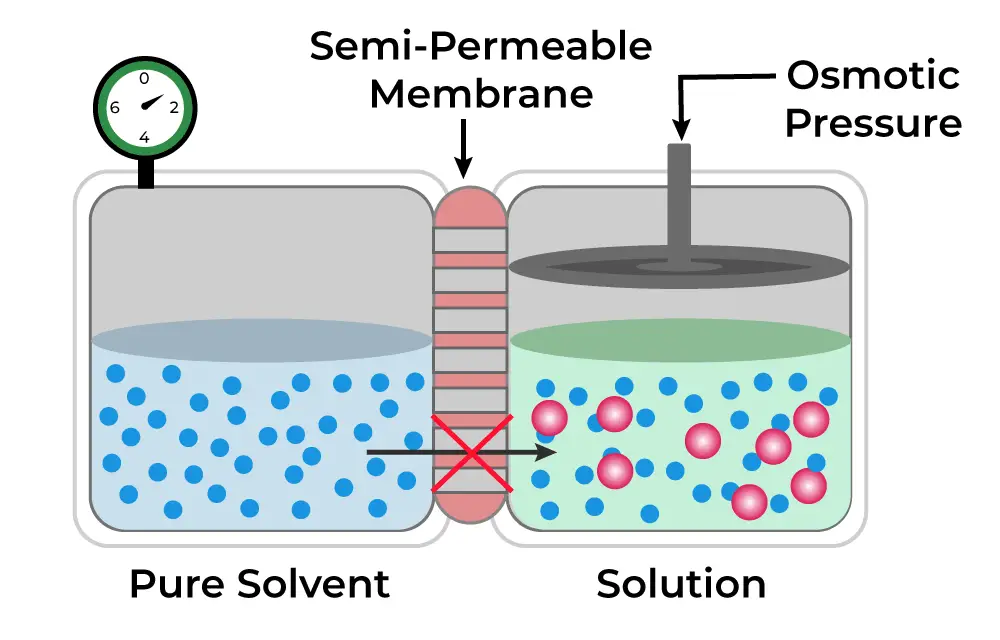
- Definition : Osmotic pressure is defined as the minimum pressure needed to halt the inward flow of pure solvent into a solution through a semi-permeable membrane. This membrane allows the passage of solvent molecules while restricting solute molecules, creating a concentration gradient that drives osmotic movement.
- Influence of Solute Concentration : The osmotic pressure of a solution is directly related to the concentration of solute particles present. As the concentration of solute increases, the osmotic pressure also rises, leading to a greater driving force for osmosis. Therefore, a more concentrated solution exerts a higher osmotic pressure, compelling solvent molecules to move toward it.
- ∏ represents the osmotic pressure,
- M denotes the molar concentration of solute particles,
- R is the gas constant, and
- T indicates the absolute temperature of the system (in Kelvin).
- Role in Biological Systems : Osmotic pressure is vital for maintaining cellular integrity and function. For example, it influences the movement of water in and out of cells, affecting cell turgidity in plants and the hydration state of animal cells. Cells must balance osmotic pressure to avoid excessive swelling or shrinking, which can compromise their viability.
Osmosis is a crucial process that significantly influences various biological and chemical systems. It facilitates the movement of water and nutrients within living organisms, maintaining cellular functions and overall health. The role of osmosis extends beyond simple water transport, impacting physiological processes critical to both plants and animals.
- Nutrient Transport : Osmosis is responsible for the absorption of essential nutrients and water from the soil into plant roots. This movement occurs through osmosis, allowing plants to transport water upward through the xylem to various parts of the plant, ensuring adequate hydration for metabolic activities.
- Cellular Homeostasis : The internal environment of living cells is stabilized by osmosis, which maintains a balance between water and intracellular fluid levels. This balance is vital for cellular functions, preventing conditions that could lead to cell damage or death.
- Turgidity Maintenance : Osmosis plays a key role in maintaining cell turgor, the pressure of the cell contents against the cell wall . Turgid cells support plant structures, enabling them to remain upright and facilitating growth. This turgidity is essential for the movement of plant parts, influencing how plants respond to environmental stimuli.
- Prevention of Desiccation : In plants, osmosis prevents the drying out of cells due to water loss during transpiration . By regulating internal water levels, plants can retain moisture, which is crucial for their survival, especially in arid environments. Additionally, osmosis helps prevent the desiccation of fruits and sporangia, ensuring successful reproduction and dispersal.
- Response to Environmental Stress : Osmosis enables plants in drought conditions to withstand water scarcity. Increased osmotic pressure within cells can help them maintain hydration, providing resilience against extreme environmental conditions.
- Water Movement and Regulation : Osmosis facilitates the diffusion of water and other cellular fluids between cells, regulating the internal movement of these substances. This process is critical for nutrient uptake, waste expulsion, and maintaining overall cellular health.
- Role in Seed Germination : During seed germination, osmosis is vital as it allows water to enter seeds, initiating metabolic processes necessary for growth. The uptake of water activates enzymes that facilitate the transformation of stored nutrients into usable forms for the developing plant.
- Physiological Mechanisms : Osmosis influences various physiological processes, including the functioning of stomata, where the movement of water molecules and the response of guard cells to osmotic pressure regulate gas exchange and transpiration rates.
- Applications in Water Purification : Beyond biological systems, osmosis has practical applications in methods such as reverse osmosis and forward osmosis. These techniques are utilized for the purification of drinking water, desalination, wastewater treatment, and even in the concentration of liquid foods.
Osmosis is a fundamental biological process that influences the movement of water across semipermeable membranes, affecting both animal and plant cells in various ways. Understanding these examples helps illustrate the critical role osmosis plays in maintaining cellular integrity and function.
- When animal cells are exposed to a hypertonic solution —where the concentration of solute is higher outside the cell—water exits the cells. This leads to a phenomenon known as crenation , where the cells shrink due to water loss. For example, red blood cells (RBCs) will shrink in size when placed in diluted blood plasma.
- Conversely, when animal cells are placed in a hypotonic solution , characterized by a lower concentration of solute, water moves into the cells. This influx can cause the cells to swell, and if the osmosis continues unchecked, it may lead to the cells bursting.
- Another illustrative example is observed in slugs; when these animals come into contact with salt, which creates a hypertonic environment, the water is drawn out from their cells. This results in the slugs shrinking, demonstrating the effects of osmotic pressure on their semi-permeable skin.
- Plant cells exhibit a different response to osmotic changes due to the presence of rigid cell walls. When placed in a hypotonic solution , plant cells take up water, leading to increased turgor pressure, which helps maintain the plant’s upright structure. This pressure is essential for supporting the plant and facilitating growth.
- However, when plant cells are exposed to a hypertonic solution , water moves out of the cells. Although the cell wall provides some structural support, the loss of water causes the cells to shrink and become flaccid. This can impact the plant’s ability to function optimally.
- A practical example of osmosis in plants is seen in the root system, where root cells absorb water from the soil through their semi-permeable membranes. This process is crucial for delivering water to various parts of the plant, influencing physiological functions.
- Additionally, the behavior of potato cells serves as a classic illustration of osmosis. When potato slices are immersed in a hypotonic solution, the cells swell as they absorb water, while placing them in a hypertonic solution results in the cells losing water and shrinking.
Osmosis and diffusion are fundamental processes that describe the movement of molecules, yet they differ significantly in their mechanisms and specific applications. Understanding these differences is crucial for comprehending various biological and chemical phenomena.
- Osmosis is a specialized form of diffusion that specifically involves the movement of solvent molecules, predominantly water, through a semipermeable membrane. This movement occurs from an area of lower solute concentration to an area of higher solute concentration.
- In contrast, diffusion refers to the general process of particles moving from a region of higher concentration to one of lower concentration. This process continues until equilibrium is established, where the concentration of particles is uniform throughout the medium.
- Osmosis typically involves only solvent molecules, such as water, which traverse the semipermeable membrane.
- Diffusion can involve a variety of particles, including solute molecules and gases, and can occur in various media, such as liquids, gases, or solids.
- Osmosis requires a semipermeable membrane that selectively allows certain molecules to pass while restricting others. This membrane plays a crucial role in determining the direction and extent of water movement.
- Diffusion can occur in the absence of any membrane. In an open system, particles can move freely without any barrier.
- In osmosis, the movement of solvent molecules is directed towards areas of higher solute concentration, contributing to the dilution of that area until equilibrium is reached.
- Diffusion involves the movement of particles from regions of higher concentration to those of lower concentration until an even distribution is achieved.
- Both osmosis and diffusion are generally passive processes that do not require energy input from the system. Osmosis is categorized as passive transport because it relies on the concentration gradient.
- While diffusion is predominantly passive, certain forms, such as facilitated diffusion , may involve specific protein channels and can require minimal energy to assist in the movement of particles.
The potato osmometers serve as effective models for studying osmosis, illustrating the principles of endosmosis and exosmosis using simple materials. This experiment provides clear insights into the movement of water through semipermeable membranes, represented by the potato tuber.
- Peeled potato
- Concentrated sugar solution
- Petri plate
- Preparation of the Potato : Start by peeling a large potato using a knife. This allows for the creation of a suitable structure to observe osmotic activity.
- Shaping the Potato : Cut the upper and lower portions of the peeled potato to ensure stability when placed on the Petri plate. This provides a flat base for the potato.
- Creating a Cavity : Use a knife to carve out a cavity in the center of the potato, extending it deep enough to hold the sugar solution but leaving a small thickness at the bottom. This bottom section acts as a selective membrane.
- Setting Up the Experiment : Position the potato on the Petri plate.
- Endosmosis Experiment : To study endosmosis, pour pure water into half of the Petri plate. Simultaneously, pour concentrated sugar solution into half of the potato cavity.
- Exosmosis Experiment : For exosmosis, place concentrated sugar solution in the Petri plate and add water to the cavity of the potato tuber.
- Marking Levels : Insert pins into the potato tuber at points A and B to indicate the initial levels of sugar solution and water in the cavity, respectively.
- Observation Period : Allow the setup to remain undisturbed for a designated time to observe any changes in the levels of the liquids.
- For Endosmosis : In the cavity of potato tuber A, observe that the level of the sugar solution increases. This indicates that water from the Petri plate is moving into the potato tuber, where there is a higher solute concentration, leading to the conclusion that water moves towards areas of lower solvent concentration.
- For Exosmosis : In the cavity of potato tuber B, note that the level of water decreases. This occurs because the water moves out of the potato tuber into the concentrated sugar solution on the Petri plate, demonstrating that water exits the cell to balance solute concentrations in the surrounding environment.
- The increase in sugar solution in tuber A confirms endosmosis, while the decrease in water in tuber B supports the concept of exosmosis, showcasing the directional movement of water based on solute concentrations.
- Ensure that the cavity is deep enough to hold the solutions while leaving an adequate thickness at the bottom of the potato to maintain structural integrity.
- Utilize a concentrated sugar solution to create a significant osmotic gradient necessary for clear observation of osmotic processes.
- Lopez MJ, Hall CA. Physiology, Osmosis. [Updated 2023 Mar 13]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557609/
- https://www.biologyonline.com/dictionary/osmosis
- https://www.geeksforgeeks.org/osmosis/
- https://biologyreader.com/study-of-osmosis-by-potato-osmometer.html
- https://www.futurelearn.com/info/courses/teaching-biology-inspiring-students-with-plants-in-science/0/steps/58750
- https://www.priyamstudycentre.com/2022/09/osmosis.html
- https://senecalearning.com/en-GB/definitions/osmosis/
- https://en.wikipedia.org/wiki/Osmosis
- https://byjus.com/biology/osmosis/#types
Related Biology Study Notes
Membrane permeability – definition, factors affects, examples, checkpoints in the cell cycle – g1, g2, metaphase (spindle) checkpoints, cell cycle – definition, phases, checkpoints, regulation, cell compartmentalization – definition, types, origins, importance, advantages, osmoregulation – definition, types, mechanism, importance, cell – structure, characteristics, types, size and shape, functions, mode of transport across plasma membrane, reactive oxygen species (ros) – definition, types, chemistry, defence, roles, latest questions.
Start Asking Questions Cancel reply
Save my name, email, and website in this browser for the next time I comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .
We've detected that you are using AdBlock Plus or some other adblocking software which is preventing the page from fully loading.
We don't have any banner, Flash, animation, obnoxious sound, or popup ad. We do not implement these annoying types of ads!
We need money to operate the site, and almost all of it comes from our online advertising.
Please add biologynotesonline.com to your ad blocking whitelist or disable your adblocking software.
- BiologyDiscussion.com
- Follow Us On:
- Google Plus
- Publish Now

Experiments on Osmosis (With Diagram)
ADVERTISEMENTS:
The below mentioned article includes a list of four simple experiments on osmosis.
1. Experiment to demonstrate the osmosis by using sheet of cellophane or goat bladder:
Requirements:
Beaker, thistle funnel, goat bladder or sheet of cellophane, thread, water and sugar solution.
1. Cover the lower opening of the glass tube with the goat bladder or sheet of cellophane and tie it with the thread.
2. Fill in the interior of the tube with molasses, a concentrated sugar solution in water.
3. Place the whole apparatus in a beaker containing water, preferably distilled water.
4. Note the level of the water in the thistle funnel and keep the apparatus to note the results.
Observations:
Level of the water in the thistle funnel increases (Fig.2).

1. Movement of water through the goat bladder or cellophane sheet into the thistle funnel takes place.
2. Water concentration in beaker is 100% while in the sugar solution it is less than this, and, therefore, the water from the region of higher concentration moves towards the region of lower concentration. The movement is through a semipermeable membrane and so the experiment shows the phenomenon of osmosis.
3. The force, with which the solution level in the tube increases, arises from the pressure exerted by the diffusion of water molecules into the tube. This pressure is called osmotic pressure.
4. Stability of the water level in the funnel indicates that water concentration in both the beakers as well as funnel is same and thus osmosis stops.
2. Experiment to demonstrate osmosis with the help of potato osmometer:
Petri-dish, water, potato, sugar solution, cork and capillary tube.
1. Take a potato tuber, remove its outer covering from one end and cut the same end flat.
2. Scoop out a cavity from the other end of the tuber running almost upto the bottom.
3. Fill the cavity with the sugar solution and fit an airtight cork fitted with a capillary tube on the upper end of the cavity (fig. 3).
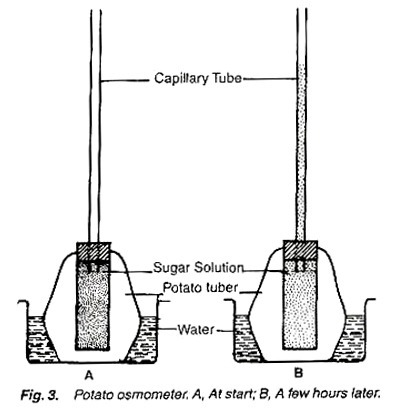
4. Place the capillary- fitted potato tuber in the water- filled petri-dish.
5. Mark the solution level in the tube and watch the experiment for some time.
After some time the level of the solution in the tube increases. Mark the level of solution when it stops to move.
The level in the capillary tube increases because of the fact that osmotic pressure of the sugar solution is higher than that of the water, and the water moves through the semipermeable membrane of potato from petri-dish into the cavity. So the experiment shows that phenomenon of osmosis.
3. Experiment to demonstrate the osmosis by the egg osmometer:
Egg membrane, dilute HCI, water through, graduated tube, sugar solution and stand.
1. Prepare an egg membrane by carefully removing waterproof shell of egg with the help of dissolving it away in dilute HCI.
2. Remove all the fat and protein-containing yellow material of the egg by making a hole on its one end.
3. Fill the sugar solution in the egg membrane through the hole and fit a graduated tube in the hole.
4. Place the complete apparatus in a water-filled trough (Fig. 4).
5. Note the level of sugar solution in the graduated tube and keep the apparatus undisturbed for some time.
Level of the sugar solution increases in the tube.
The level in the tube increases because of the fact that osmotic pressure of the sugar solution in the egg membrane is higher than that of water, and so the water from the trough passes through the egg membrane into the sugar solution thus increasing its level. Egg membrane is a semipermeable membrane.
4. Experiment to demonstrate the phenomenon of exosmosis and endosmosis:
Potato tubers (2), knife, conc. sugar solution, water, pin, beakers (2).
1. Remove the outer skin of the tubers and cut their one end flat with a sharp knife.
2. Scoop out a cavity from the other end of the tuber running almost upto the bottom as in experiment No. 14.
3. Fill the concentrated solution of sugar in the cavity of one tuber, and water in the other.
4. Mark the level of the sugar solution and water in the cavities with the help of pins.
5. Place the potato containing sugar solution in a beaker containing water, and the another potato containing water in its cavity in the beaker containing sugar solution (Fig. 5).
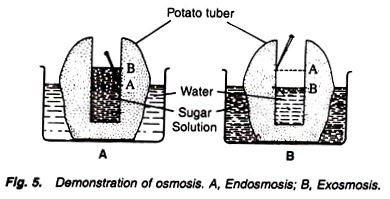
6. Keep and observe experiment for some time.
The level in the cavity containing sugar solution increases while the level decreases in the another tuber, i.e., in the cavity filled with water.
The level of the sugar solution in the first tuber increases because of the fact that water moves from the beaker into the cavity through the semipermeable membrane of potato. Thus it shows the phenomenon of endosmosis.
The level of the water in the second tuber decreases because of the fact that water moves from the cavity into the beaker through the semipermeable membrane of potato tuber. Thus it shows the phenomenon of exosmosis.
Related Articles:
- Experiment on Osmosis in Potatoes | Botany
- Top 6 Experiments on Osmosis (With Diagram)
Experiment , Chemistry , Osmosis , Experiments on Osmosis
- Anybody can ask a question
- Anybody can answer
- The best answers are voted up and rise to the top
Forum Categories
- Animal Kingdom
- Biodiversity
- Biological Classification
- Biology An Introduction 11
- Biology An Introduction
- Biology in Human Welfare 175
- Biomolecules
- Biotechnology 43
- Body Fluids and Circulation
- Breathing and Exchange of Gases
- Cell- Structure and Function
- Chemical Coordination
- Digestion and Absorption
- Diversity in the Living World 125
- Environmental Issues
- Excretory System
- Flowering Plants
- Food Production
- Genetics and Evolution 110
- Human Health and Diseases
- Human Physiology 242
- Human Reproduction
- Immune System
- Living World
- Locomotion and Movement
- Microbes in Human Welfare
- Mineral Nutrition
- Molecualr Basis of Inheritance
- Neural Coordination
- Organisms and Population
- Photosynthesis
- Plant Growth and Development
- Plant Kingdom
- Plant Physiology 261
- Principles and Processes
- Principles of Inheritance and Variation
- Reproduction 245
- Reproduction in Animals
- Reproduction in Flowering Plants
- Reproduction in Organisms
- Reproductive Health
- Respiration
- Structural Organisation in Animals
- Transport in Plants
- Trending 14
Privacy Overview
- JEE Main Exam
- JEE Advanced Exam
- BITSAT Exam
- View All Engineering Exams
- Colleges Accepting B.Tech Applications
- Top Engineering Colleges in India
- Engineering Colleges in India
- Engineering Colleges in Tamil Nadu
- Engineering Colleges Accepting JEE Main
- Top IITs in India
- Top NITs in India
- Top IIITs in India
- JEE Main College Predictor
- JEE Main Rank Predictor
- MHT CET College Predictor
- AP EAMCET College Predictor
- GATE College Predictor
- KCET College Predictor
- JEE Advanced College Predictor
- View All College Predictors
- JEE Advanced Cutoff
- JEE Main Cutoff
- GATE Registration 2025
- JEE Main Syllabus 2025
- Download E-Books and Sample Papers
- Compare Colleges
- B.Tech College Applications
- JEE Main Question Papers
- View All Management Exams
Colleges & Courses
- Top MBA Colleges in India
- MBA College Admissions
- MBA Colleges in India
- Top IIMs Colleges in India
- Top Online MBA Colleges in India
- MBA Colleges Accepting XAT Score
- BBA Colleges in India
- XAT College Predictor 2025
- SNAP College Predictor
- NMAT College Predictor
- MAT College Predictor 2024
- CMAT College Predictor 2025
- CAT Percentile Predictor 2024
- CAT 2024 College Predictor
- Top MBA Entrance Exams 2024
- SNAP Registration
- GD Topics for MBA
- CAT 2024 Admit Card
- Download Helpful Ebooks
- List of Popular Branches
- QnA - Get answers to your doubts
- IIM Fees Structure
- AIIMS Nursing
- Top Medical Colleges in India
- Top Medical Colleges in India accepting NEET Score
- Medical Colleges accepting NEET
- List of Medical Colleges in India
- List of AIIMS Colleges In India
- Medical Colleges in Maharashtra
- Medical Colleges in India Accepting NEET PG
- NEET College Predictor
- NEET PG College Predictor
- NEET MDS College Predictor
- NEET Rank Predictor
- DNB PDCET College Predictor
- NEET Syllabus 2025
- NEET Study Material 2024
- NEET Cut off
- NEET Exam Date 2025
- Download Helpful E-books
- Colleges Accepting Admissions
- Top Law Colleges in India
- Law College Accepting CLAT Score
- List of Law Colleges in India
- Top Law Colleges in Delhi
- Top NLUs Colleges in India
- Top Law Colleges in Chandigarh
- Top Law Collages in Lucknow
Predictors & E-Books
- CLAT College Predictor
- MHCET Law ( 5 Year L.L.B) College Predictor
- AILET College Predictor
- Sample Papers
- Compare Law Collages
- Careers360 Youtube Channel
- CLAT Syllabus 2025
- Free CLAT Practice Test
- NID DAT Exam
- Pearl Academy Exam
Predictors & Articles
- NIFT College Predictor
- UCEED College Predictor
- NID DAT College Predictor
- NID DAT 2025
- NID DAT Syllabus 2025
- Design Colleges in India
- Top NIFT Colleges in India
- Fashion Design Colleges in India
- Top Interior Design Colleges in India
- Top Graphic Designing Colleges in India
- Fashion Design Colleges in Delhi
- Fashion Design Colleges in Mumbai
- Top Interior Design Colleges in Bangalore
- NIFT Cutoff
- NIFT Fees Structure
- NIFT Syllabus 2025
- Free Sample Papers
- Free Design E-books
- List of Branches
- Careers360 Youtube channel
- IPU CET BJMC 2024
- JMI Mass Communication Entrance Exam 2024
- IIMC Entrance Exam 2024
- MICAT Exam 2025
- Media & Journalism colleges in Delhi
- Media & Journalism colleges in Bangalore
- Media & Journalism colleges in Mumbai
- List of Media & Journalism Colleges in India
- Free Ebooks
- CA Intermediate
- CA Foundation
- CS Executive
- CS Professional
- Difference between CA and CS
- Difference between CA and CMA
- CA Full form
- CMA Full form
- CS Full form
- CA Salary In India

Top Courses & Careers
- Bachelor of Commerce (B.Com)
- Master of Commerce (M.Com)
- Company Secretary
- Cost Accountant
- Charted Accountant
- Credit Manager
- Financial Advisor
- Top Commerce Colleges in India
- Top Government Commerce Colleges in India
- Top Private Commerce Colleges in India
- Top M.Com Colleges in Mumbai
- Top B.Com Colleges in India
- IT Colleges in Tamil Nadu
- IT Colleges in Uttar Pradesh
- MCA Colleges in India
- BCA Colleges in India
Quick Links
- Information Technology Courses
- Programming Courses
- Web Development Courses
- Data Analytics Courses
- Big Data Analytics Courses
- RUHS Pharmacy Admission Test
- Top Pharmacy Colleges in India
- Pharmacy Colleges in Pune
- Pharmacy Colleges in Mumbai
- Colleges Accepting GPAT Score
- Pharmacy Colleges in Lucknow
- List of Pharmacy Colleges in Nagpur
- GPAT Result
- GPAT 2024 Admit Card
- GPAT Question Papers
- NCHMCT JEE 2025
- Mah BHMCT CET
- Top Hotel Management Colleges in Delhi
- Top Hotel Management Colleges in Hyderabad
- Top Hotel Management Colleges in Mumbai
- Top Hotel Management Colleges in Tamil Nadu
- Top Hotel Management Colleges in Maharashtra
- B.Sc Hotel Management
- Hotel Management
- Diploma in Hotel Management and Catering Technology
Diploma Colleges
- Top Diploma Colleges in Maharashtra
- UPSC IAS 2024
- SSC CGL 2024
- IBPS RRB 2024
- Previous Year Sample Papers
- Free Competition E-books
- Sarkari Result
- QnA- Get your doubts answered
- UPSC Previous Year Sample Papers
- CTET Previous Year Sample Papers
- SBI Clerk Previous Year Sample Papers
- NDA Previous Year Sample Papers
Upcoming Events
- NDA 2 Admit card 2024
- SSC CGL Admit card 2024
- CDS 2 Admit card 2024
- UGC NET Admit card 2024
- HP TET Result 2024
- SSC CHSL Result 2024
- UPTET Notification 2024
- SBI PO Notification 2024
Other Exams
- SSC CHSL 2024
- UP PCS 2024
- UGC NET 2024
- RRB NTPC 2024
- IBPS PO 2024
- IBPS Clerk 2024
- IBPS SO 2024
- CBSE Class 10th
- CBSE Class 12th
- UP Board 10th
- UP Board 12th
- Bihar Board 10th
- Bihar Board 12th
Top Schools
- Top Schools in India
- Top Schools in Delhi
- Top Schools in Mumbai
- Top Schools in Chennai
- Top Schools in Hyderabad
- Top Schools in Kolkata
- Top Schools in Pune
- Top Schools in Bangalore
Products & Resources
- JEE Main Knockout April
- RD Sharma Solutions
- Navodaya Vidyalaya Admission 2024-25
NCERT Study Material
- NCERT Notes
- NCERT Books
- NCERT Syllabus
- NCERT Solutions
- NCERT Solutions for Class 12
- NCERT Solutions for Class 11
- NCERT solutions for Class 10
- Top University in USA
- Top University in Canada
- Top University in Ireland
- Top Universities in UK
- Top Universities in Australia
- Best MBA Colleges in Abroad
- Business Management Studies Colleges
Top Countries
- Study in USA
- Study in UK
- Study in Canada
- Study in Australia
- Study in Ireland
- Study in Germany
- Study in China
- Study in Europe
Student Visas
- Student Visa Canada
- Student Visa UK
- Student Visa USA
- Student Visa Australia
- Student Visa Germany
- Student Visa New Zealand
- Student Visa Ireland
- CUET PG 2025
- UP B.Ed JEE 2024
- TS EDCET Exam
- IIT JAM 2025
- AP PGCET Exam
- Universities in India
- Top Universities in India 2024
- Top Colleges in India
- Top Universities in Uttar Pradesh 2024
- Top Universities in Bihar
- Top Universities in Madhya Pradesh 2024
- Top Universities in Tamil Nadu 2024
- Central Universities in India
- CUET DU Cut off 2024
- IGNOU Date Sheet 2024
- CUET DU CSAS Portal 2024
- CUET 2025 Syllabus
- CUET PG Syllabus 2025
- CUET Participating Universities 2025
- CUET Previous Year Question Paper
- IGNOU Result 2024
- E-Books and Sample Papers
- CUET College Predictor 2024
- CUET Exam Date 2025
- CUET Cut Off 2024
- NIRF Ranking 2024
- IGNOU Exam Form 2024
- CUET Syllabus
- CUET Counselling 2025
Engineering Preparation
- Knockout JEE Main 2024
- Test Series JEE Main 2024
- JEE Main 2024 Rank Booster
Medical Preparation
- Knockout NEET 2024
- Test Series NEET 2024
- Rank Booster NEET 2024
Online Courses
- JEE Main One Month Course
- NEET One Month Course
- IBSAT Free Mock Tests
- IIT JEE Foundation Course
- Knockout BITSAT 2024
- Career Guidance Tool
Top Streams
- IT & Software Certification Courses
- Engineering and Architecture Certification Courses
- Programming And Development Certification Courses
- Business and Management Certification Courses
- Marketing Certification Courses
- Health and Fitness Certification Courses
- Design Certification Courses
Specializations
- Digital Marketing Certification Courses
- Cyber Security Certification Courses
- Artificial Intelligence Certification Courses
- Business Analytics Certification Courses
- Data Science Certification Courses
- Cloud Computing Certification Courses
- Machine Learning Certification Courses
- View All Certification Courses
- UG Degree Courses
- PG Degree Courses
- Short Term Courses
- Free Courses
- Online Degrees and Diplomas
- Compare Courses
Top Providers
- Coursera Courses
- Udemy Courses
- Edx Courses
- Swayam Courses
- upGrad Courses
- Simplilearn Courses
- Great Learning Courses
Study Of Osmosis By Potato Osmometer: Diagram, Experiment
To study by demonstrating the osmosis process by potato osmometer.
Osmosis forms the base of understanding the flow of water through semipermeable membranes in organisms. The paper considers the process of osmosis using a potato osmometer, an apparatus that clearly shows this particular process. In the experiment, we will see how water is moved from high to low water potential across a semipermeable membrane and how different solutions affect this process.
Latest: NEET 2024 Paper Analysis and Answer Key
Don't Miss: Most scoring concepts for NEET | NEET papers with solutions
New: NEET Syllabus 2025 for Physics, Chemistry, Biology
NEET Important PYQ & Solutions: Physics | Chemistry | Biology | NEET PYQ's (2015-24)
What Is Osmosis?
Osmosis is the flow of solvent molecules across the semipermeable membrane from areas of higher water potential to those with lower water potential and vice versa. It is a continued movement from high water potential to low until the concentration of solutes on either side of the membrane level out.
Solvent And Solute
In osmosis, the solvent refers to the liquid that crosses a semipermeable membrane, while the solute includes particles dissolved in the latter. The two of them make up a solution.
What Is The Solution?
A solution is a uniform mixture of a solute and a solvent. Its property relies on the concentration of the solute and the solvent.
Solution Types
The different types of solutions:
Hypertonic Solution
A solution with high concentrations of solutes. Now, when cells are put in a hypertonic solution, since it is hypertonic to them, because of osmosis, the water rushes out of the cells, and they shrink.
Hypotonic Solution
A solution with low concentrations of solutes. Now, if cells are put into a hypotonic solution, then, because of osmosis, water will rush into the cell and it will swell up and become turgid.
Isotonic Solution
A solution where the concentration of the solute is equal on both sides of the membrane. Cells in an isotonic solution retain their shape because there is no net movement of water.
Material Required
Fresh large-sized potato tuber
Sucrose solution of 20% concentration
Scalpel or blade
Bell pin needle marked with waterproof ink
Cut the potato tuber into two halves using a scalpel or blade. Remove the skin and then cut these halves into square pieces.
Scoop out a small cavity from the mid-region of the potato tuber having a minimum thickness at the base. The cavity may be square or circular.
Fill half the cavity with freshly prepared 20% sucrose solution and put a pin into the cavity so that its mark coincides with the level of sucrose solution.
Place the potato osmometer in a petri dish or beaker containing water; the level is to be such that 75 % of the potato osmometer will be submerged in the water.
Allow to remain undisturbed for about 1 hour.
Observe and note the level of the sucrose solution in the osmometer at the end of the experiment.
Repeat the experiment with the cavity filled with water and the sucrose solution in the Petri dish or beaker.
Observation
After an appropriate length of time, the sugar solution inside the potato osmometer will rise and may also become coloured. This shows that on account of osmosis, the water moves inside the osmometer.
This rise in the level of sucrose solution in the osmometer is, thus, a case of endosmosis, where the water from the external beaker enters the sucrose solution within the potato. This process thus creates a water potential gradient between the external water and the osmometer. Since water can enter the sucrose solution through the selectively permeable membrane of the osmometer, separated by living potato cells, this process is explained as osmosis.
Recommended video on Study Of Osmosis By Potato Osmometer
Frequently Asked Questions (FAQs)
Osmosis is defined as the flow of water across a semipermeable membrane from an area of high potential to that with low potential. It helps in maintaining cell turgor and provides the skeletal framework necessary to maintain the water balance in living organisms.
The potato osmometer is the one that shows the movement of water through the potato's semipermeable membrane into a solution with a higher concentration of solutes, hence osmotic in action.
Hypertonic solutions cause the shrinking of cells, hypotonic solutions make them swell, and isotonic solutions don't result in the net movement of water; thus, the shape remains the same.
Temperature does affect the rate of osmosis; thus, by keeping this factor constant, any changes observed will be due to the solute concentration and not to extraneous variables.
These principles can be further researched in the fields of medicine and agriculture by studying the flow of water through the cells and thus refining the practices associated with the hydration and solute management of cells.
16 Oct'24 01:35 PM
15 Oct'24 05:34 PM
19 Sep'24 12:30 PM
19 Sep'24 12:05 PM
19 Sep'24 11:43 AM
19 Sep'24 11:32 AM
18 Sep'24 06:51 PM
18 Sep'24 06:40 PM
18 Sep'24 06:17 PM
18 Sep'24 06:15 PM
- Latest Articles
- Popular Articles
Download Careers360 App
All this at the convenience of your phone.
Regular Exam Updates
Best College Recommendations
College & Rank predictors
Detailed Books and Sample Papers
Question and Answers
Scan and download the app

IMAGES
VIDEO
COMMENTS
It is a common experiment to demonstrate both endosmosis and exosmosis using a potato. Using a potato Osmoscope, we can study osmosis in a living system. Rule of osmosis: Water moves from high solvent to low solvent concentration. In other words, water moves from low solute to high solute concentration.
To study and analyze the process of osmosis using potato osmometer. Explore more in detail about Osmosis and Diffusion of water only at BYJU'S.
The potato osmometers serve as effective models for studying osmosis, illustrating the principles of endosmosis and exosmosis using simple materials. This experiment provides clear insights into the movement of water through semipermeable membranes, represented by the potato tuber.
The below mentioned article includes a list of four simple experiments on osmosis. 1. Experiment to demonstrate the osmosis by using sheet of cellophane or goat bladder: Requirements: Beaker, thistle funnel, goat bladder or sheet of cellophane, thread, water and sugar solution.
Gatorade. Waste Disposal. Solutions, colloids, and suspensions provided should be kept in or returned to their containers for future use. \ (\ce {NaCl}\) solutions can go down the drain. Used potatoes can go in the trash. Learning Objectives. Recognize a solution and its composition of a solute and solvent.
In the potato osmometer experiment, the cells of the potato allow the entry of water molecules, i.e. the cells exhibit endosmosis. The water molecules reach the sugar solution kept inside the potato osmometer, causing a rise in the level of the sugar solution.
The paper considers the process of osmosis using a potato osmometer, an apparatus that clearly shows this particular process. In the experiment, we will see how water is moved from high to low water potential across a semipermeable membrane and how different solutions affect this process.
Experiment: Osmosis in Potatoes . Distribute the following materials to each pair: . 1 Potato Activity Sheet . 1 250mL bottle of distilled water . 2 5 oz. cups, marked to 30 mL . 1 container of salt . 1 spoon .
Aim: To demonstrate osmosis by potato osmometer. Principle: Osmosis is a common physical process observed in living cells and tissues of all organisms. It is defined as the movement of molecules of solvent from a region of its higher concentration to a region of its lower concentration across a selectively permeable membrane,
paper towels. Using the waterproof pen, label each tube with the name of one of the solutions. Place the boiling tubes in the rack. Dry a potato strip carefully by blotting it with a paper towel. Measure its mass on the balance. Place the potato strip into one of the tubes.