Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 30 July 2020

The current and future landscape of dialysis
- Jonathan Himmelfarb ORCID: orcid.org/0000-0002-3319-1224 1 , 2 ,
- Raymond Vanholder ORCID: orcid.org/0000-0003-2633-1636 3 ,
- Rajnish Mehrotra ORCID: orcid.org/0000-0003-2833-067X 1 , 2 &
- Marcello Tonelli ORCID: orcid.org/0000-0002-0846-3187 4
Nature Reviews Nephrology volume 16 , pages 573–585 ( 2020 ) Cite this article
90k Accesses
295 Citations
130 Altmetric
Metrics details
- Haemodialysis
- Health care economics
- Health services
- Medical ethics
The development of dialysis by early pioneers such as Willem Kolff and Belding Scribner set in motion several dramatic changes in the epidemiology, economics and ethical frameworks for the treatment of kidney failure. However, despite a rapid expansion in the provision of dialysis — particularly haemodialysis and most notably in high-income countries (HICs) — the rate of true patient-centred innovation has slowed. Current trends are particularly concerning from a global perspective: current costs are not sustainable, even for HICs, and globally, most people who develop kidney failure forego treatment, resulting in millions of deaths every year. Thus, there is an urgent need to develop new approaches and dialysis modalities that are cost-effective, accessible and offer improved patient outcomes. Nephrology researchers are increasingly engaging with patients to determine their priorities for meaningful outcomes that should be used to measure progress. The overarching message from this engagement is that while patients value longevity, reducing symptom burden and achieving maximal functional and social rehabilitation are prioritized more highly. In response, patients, payors, regulators and health-care systems are increasingly demanding improved value, which can only come about through true patient-centred innovation that supports high-quality, high-value care. Substantial efforts are now underway to support requisite transformative changes. These efforts need to be catalysed, promoted and fostered through international collaboration and harmonization.
The global dialysis population is growing rapidly, especially in low-income and middle-income countries; however, worldwide, a substantial number of people lack access to kidney replacement therapy, and millions of people die of kidney failure each year, often without supportive care.
The costs of dialysis care are high and will likely continue to rise as a result of increased life expectancy and improved therapies for causes of kidney failure such as diabetes mellitus and cardiovascular disease.
Patients on dialysis continue to bear a high burden of disease, shortened life expectancy and report a high symptom burden and a low health-related quality of life.
Patient-focused research has identified fatigue, insomnia, cramps, depression, anxiety and frustration as key symptoms contributing to unsatisfactory outcomes for patients on dialysis.
Initiatives to transform dialysis outcomes for patients require both top-down efforts (that is, efforts that promote incentives based on systems level policy, regulations, macroeconomic and organizational changes) and bottom-up efforts (that is, patient-led and patient-centred advocacy efforts as well as efforts led by individual teams of innovators).
Patients, payors, regulators and health-care systems increasingly demand improved value in dialysis care, which can only come about through true patient-centred innovation that supports high-quality, high-value care.
Similar content being viewed by others
Epidemiology of haemodialysis outcomes

Epidemiology of peritoneal dialysis outcomes
Kidney disease trials for the 21st century: innovations in design and conduct
Introduction.
Haemodialysis as a treatment for irreversible kidney failure arose from the pioneering efforts of Willem Kolff and Belding Scribner, who together received the 2002 Albert Lasker Clinical Medical Research Award for this accomplishment. Kolff treated his first patient with an artificial kidney in 1943 — a young woman who was dialysed 12 times successfully but ultimately died because of vascular access failure. By 1945, Kolff had dialysed 15 more patients who did not survive, when Sofia Schafstadt — a 67-year-old woman who had developed acute kidney injury — recovered, becoming the first long-term survivor after receipt of dialysis. In 1960, Belding Scribner, Wayne Quinton and colleagues at the University of Washington, WA, USA, designed shunted cannulas, which prevented the destruction of blood vessels and enabled repeated haemodialysis sessions. The first patient who received long-term treatment (named Clyde Shields) lived a further 11 years on haemodialysis. In their writings, both Kolff and Scribner eloquently described being motivated by their perception of helplessness as physicians who had little to offer for the care of young patients who were dying of uraemia and stated that the goal of dialysis was to achieve full rehabilitation to an enjoyable life 1 .
The potential to scale the use of dialysis to treat large numbers of patients with kidney failure created great excitement. At the 1960 meeting of the American Society for Artificial Internal Organs (ASAIO), Scribner introduced Clyde Shields to physicians interested in dialysis, and Quinton demonstrated fabrication of the shunt. The following decade saw rapid gains in our understanding of kidney failure, including the discovery of uraemia-associated atherogenesis and metabolic bone disease, and in virtually every aspect of haemodialysis, including improvements in dialyser technology, dialysate composition, materials for haemocompatibility and water purification systems. The Scribner–Quinton shunt rapidly became an historical artefact once Brescia and colleagues developed the endogenous arteriovenous fistula in 1966 (ref. 2 ), and prosthetic subcutaneous interpositional ‘bridge’ grafts were developed shortly thereafter. Concomitant with these pioneering efforts, in 1959, peritoneal dialysis (PD) was first used successfully to sustain life for 6 months. Within 2 years a long-term PD programme was established in Seattle, WA, USA, and within 3 years the first automated PD cycler was developed 3 .
In 1964, Scribner’s presidential address to the ASAIO described emerging ethical issues related to dialysis, including considerations for patient selection, patient self-termination of treatment as a form of suicide, approaches to ensure death with dignity and selection criteria for transplantation 4 . Indeed, the process of selecting who would receive dialysis contributed to the emergence of the field of bioethics. The early success of dialysis paradoxically created social tensions, as access to this life-sustaining therapy was rationed by its availability and the ‘suitability’ of patients. In the early 1970s, haemodialysis remained a highly specialized therapy, available to ~10,000 individuals, almost exclusively in North America and Europe, with a high frequency of patients on home haemodialysis. In a portentous moment, Shep Glazer, an unemployed salesman, was dialysed in a live demonstration in front of the US Congress House Ways and Means Committee. Soon thereafter, in October 1972, an amendment to the Social Security Act creating Medicare entitlement for end-stage renal disease (now known as kidney failure), for both dialysis and kidney transplantation, was passed by Congress and signed into law by President Nixon.
The resulting expansion of dialysis, previously described as “from miracle to mainstream” 5 , set in motion dramatic changes 6 , including the development of a for-profit outpatient dialysis provider industry; relaxation of stringent patient selection for dialysis eligibility in most HICs; a move away from home towards in-centre dialysis; efforts on the part of single payors such as Medicare in the USA to restrain per-patient costs through the introduction of bundled payments and the setting of composite rates; the development of quality indicators — such as adequate urea clearance per treatment — that were readily achievable but are primarily process rather than outcome measures; consolidation of the dialysis industry, particularly in the USA owing to economies of scale, eventually resulting in a duopoly of dialysis providers; the development of joint ventures and other forms of partnerships between dialysis providers and nephrologists; the globalization of dialysis, which is now available, albeit not necessarily accessible or affordable in many low-income and middle-income countries (LMICs); and finally, a dramatic slowing in the rate of true patient-centred innovation, with incremental gains in dialysis safety and efficiency replacing the pioneering spirit of the early innovators.
The population of patients receiving dialysis continues to grow rapidly, especially in LMICs, as a result of an increase in the availability of dialysis, population ageing, increased prevalence of hypertension and diabetes mellitus, and toxic environmental exposures. However, despite the global expansion of dialysis, notable regional differences exist in the prevalence of different dialysis modalities and in its accessibility. Worldwide, a substantial number of people do not have access to kidney replacement therapy (KRT), resulting in millions of deaths from kidney failure each year. Among populations with access to dialysis, mortality remains high and outcomes suboptimal, with high rates of comorbidities and poor health-related quality of life. These shortcomings highlight the urgent need for innovations in the dialysis space to increase accessibility and improve outcomes, with a focus on those that are a priority to patients. This Review describes the current landscape of dialysis therapy from an epidemiological, economic, ethical and patient-centred framework, and provides examples of initiatives that are aimed at stimulating innovations in dialysis and transform the field to one that supports high-quality, high-value care.
Epidemiology of dialysis
Kidney failure is defined by a glomerular filtration rate <15 ml/min/1.73 m 2 (ref. 7 ) and may be treated using KRT (which refers to either dialysis or transplantation) or with supportive care 8 . The global prevalence of kidney failure is uncertain, but was estimated to be 0.07%, or approximately 5.3 million people in 2017 (ref. 9 ), with other estimates ranging as high as 9.7 million. Worldwide, millions of people die of kidney failure each year owing to a lack of access to KRT 10 , often without supportive care. Haemodialysis is costly, and current recommendations therefore suggest that haemodialysis should be the lowest priority for LMICs seeking to establish kidney care programmes. Rather, these programmes should prioritize other approaches, including treatments to prevent or delay kidney failure, conservative care, living donor kidney transplantation and PD 11 . Nonetheless, haemodialysis is the most commonly offered form of KRT in LMICs, as well as in high-income countries (HICs) 12 , and continued increases in the uptake of haemodialysis are expected worldwide in the coming decades. Here, we review the basic epidemiology of kidney failure treated with long-term dialysis and discuss some of the key epidemiological challenges of the future (Fig. 1a ).
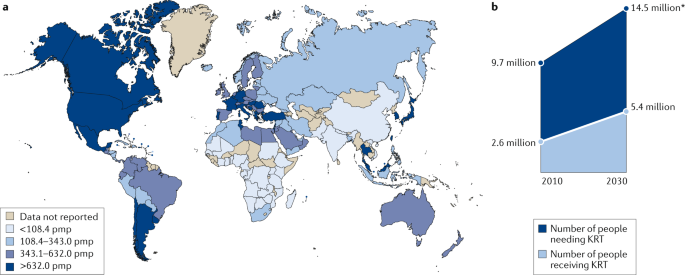
Growth is continuously outpacing the capacity of kidney replacement therapy (KRT), defined as maintenance dialysis or kidney transplant, especially in low-income and middle-income countries. a | Global prevalence of chronic dialysis. b | Estimated worldwide need and projected capacity for KRT by 2030. pmp, per million population. Adapted with permission from the ISN Global Kidney Health Atlas 2019.
Prevalence of dialysis use
Prevalence of haemodialysis.
Worldwide, approximately 89% of patients on dialysis receive haemodialysis; the majority (>90%) of patients on haemodialysis live in HICs or the so-called upper middle-income countries such as Brazil and South Africa 12 , 13 . The apparent prevalence of long-term dialysis varies widely by region but correlates strongly with national income 14 . This variation in prevalence in part reflects true differences in dialysis use 12 , 15 but also reflects the fact that wealthier countries are more likely than lower income countries to have comprehensive dialysis registries. Of note, the prevalence of haemodialysis is increasing more rapidly in Latin America (at a rate of ~4% per year) than in Europe or the USA (both ~2% per year), although considerable variation between territories exists in all three of these regions, which again correlates primarily (but not exclusively) with wealth 16 , 17 . The prevalence of haemodialysis varies widely across South Asia, with relatively high prevalence (and rapid growth) in India and lower prevalence in Afghanistan and Bangladesh 18 . Limited data are available on the prevalence of dialysis therapies in sub-Saharan Africa 19 . A 2017 report suggests that haemodialysis services were available in at least 34 African countries as of 2017, although haemodialysis was not affordable or accessible to the large majority of resident candidates 13 .
Prevalence of peritoneal dialysis
Worldwide, PD is less widely available than haemodialysis. In a 2017 survey of 125 countries, PD was reportedly available in 75% of countries whereas haemodialysis was available in 96% 20 . In 2018, an estimated 11% of patients receiving long-term dialysis worldwide were treated with PD; a little over half of these patients were living in China, Mexico, the USA and Thailand 21 .
Large variation exists between territories in the relative use of PD for treating kidney failure; in Hong Kong for example, >80% of patients on dialysis receive PD, whereas in Japan this proportion is <5% 22 . This variation is, in part, determined by governmental policies and the density of haemodialysis facilities 23 . In some countries such as the USA, rates of PD utilization also vary by ethnicity with African Americans and Hispanics being much less likely than white Americans to receive PD 24 . Disparate secular trends in PD use are also evident, with rapid growth in the use of PD in some regions such as the USA, China and Thailand and declining or unchanging levels of PD use in other regions, for example, within Western Europe 22 . As for haemodialysis, access to PD is poor in many LMICs for a variety of reasons, as comprehensively discussed elsewhere 25 .
Incidence of dialysis use
Following a rapid increase in dialysis use over a period of approximately two decades, the incidence of dialysis initiation in most HICs reached a peak in the early 2000s and has remained stable or slightly decreased since then 22 , 26 , 27 . Extrapolation of prevalence data from LMICs suggests that the incidence of dialysis initiation seems to be steadily increasing in LMICs 10 , 28 , 29 , 30 , with further increases expected over the coming decades. However, incidence data in LMICs are less robust than prevalence data, although neither reflect the true demand for KRT given the lack of reporting.
Of note, the incidence of dialysis initiation in HICs is consistently 1.2-fold to 1.4-fold higher for men than for women, despite an apparently higher risk of chronic kidney disease (CKD) in women 31 . Whether this finding reflects physician or health system bias, different preferences with regard to KRT, disparities in the competing risk of death, variation in rates of kidney function loss in women versus men, or other reasons is unknown and requires further study. Few data describe the incidence of haemodialysis by sex in LMICs.
Dialysis outcomes
Mortality is very high among patients on dialysis, especially in the first 3 months following initiation of haemodialysis treatment. Approximately one-quarter of patients on haemodialysis die within a year of initiating therapy in HICs, and this proportion is even higher in LMICs 32 , 33 , 34 . Over the past two decades, reductions in the relative and absolute risk of mortality have seemingly been achieved for patients on haemodialysis. Data suggest that relative gains in survival may be greater for younger than for older individuals; however, absolute gains seem to be similar across age groups 35 . Although controversial, improvements in mortality risk seem to have been more rapid among patients on dialysis than for the general population 36 , suggesting that better care of patients receiving dialysis treatments rather than overall health gains might be at least partially responsible for these secular trends. The factors responsible for these apparent trends have not been confirmed, but could include better management of comorbidities, improvements in the prevention or treatment of dialysis-related complications such as infection, and/or better care prior to the initiation of dialysis (which may translate into better health following dialysis initiation). Historically, although short-term mortality was lower for patients treated with PD than for those treated with haemodialysis, the long-term mortality risk was higher with PD 37 , 38 . In the past two decades, the reduction in mortality risk has been greater for patients treated with PD than with haemodialysis, such that in most regions the long-term survival of patients treated with PD and haemodialysis are now similar 39 , 40 , 41 .
Despite these improvements, mortality remains unacceptably high among patients on dialysis and is driven by cardiovascular events and infection. For example, a 2019 study showed that cardiovascular mortality among young adults aged 22–29 years with incident kidney failure was 143–500-fold higher than that of otherwise comparable individuals without kidney failure, owing to a very high burden of cardiovascular risk factors 42 . The risk of infection is also markedly greater among patients on dialysis than in the general population, in part driven by access-related infections in patients on haemodialysis with central venous catheters and peritonitis-related infections in patients on PD 43 , 44 , 45 , 46 , 47 . Hence, strategies to reduce the risk of infection associated with dialysis access should continue to be a high clinical priority.
The risk of mortality among patients on dialysis seems to be influenced by race. In the USA, adjusted mortality is lower for African American patients than for white patients on dialysis, although there is a significant interaction with age such that this observation held only among older adults, and the converse is actually true among younger African American patients aged 18 to 30 years 48 . A similar survival advantage is observed among Black patients compared with white patients or patients of Asian heritage on haemodialysis in the Netherlands 49 . In Canada, dialysis patients of indigenous descent have higher adjusted mortality, and patients of South Asian or East Asian ethnicity have lower adjusted mortality than that of white patients. In addition, between-region comparisons indicate that mortality among incident dialysis patients is substantially lower for Japan than for other HICs. Whether this difference is due to ethnic origin, differences in health system practices, a combination of these factors or other, unrelated factors is unknown 30 . No consistent evidence exists to suggest that mortality among incident adult dialysis patients varies significantly by sex 50 , 51 , 52 .
Other outcomes
Hospitalization, inability to work and loss of independent living are all markedly more common among patients on dialysis than in the general population 53 , 54 , 55 . In contrast to the modest secular improvements in mortality achieved for patients on dialysis, health-related quality of life has remained unchanged for the past two decades and is substantially lower than that of the general population, due in part to high symptom burden 56 , 57 , 58 , 59 . Depression is also frequent among patients on dialysis 60 , and factors such as high pill burden 61 , the need to travel to dialysis sessions and pain associated with vascular access puncture all affect quality of life 62 .
Future epidemiological challenges
The changing epidemiology of kidney failure is likely to present several challenges for the optimal management of these patients. For example, the ageing global population together with continuing increases in the prevalence of key risk factors for the development of kidney disease, such as diabetes mellitus and hypertension, mean that the incidence, prevalence and costs of kidney failure will continue to rise for the foreseeable future. This increased demand for KRT will undoubtedly lead to an increase in the uptake of haemodialysis, which will pose substantial economic challenges for health systems worldwide. Moreover, as growth in demand seems to be outpacing increases in KRT capacity, the number of deaths as a result of kidney failure is expected to rise dramatically (Fig. 1b ).
The same risk factors that drive the development of kidney disease will also increase the prevalence of multimorbidities within the dialysis population. These comorbidities will in turn require effective management in addition to the management of kidney failure per se 63 and will require technical innovations of dialysis procedures, as well as better evidence to guide the management of comorbidities in the dialysis population.
Finally, the particularly rapid increases in the incidence and prevalence of kidney failure among populations in LMICs will place considerable strain on the health systems of these countries. The associated increases in mortality resulting from a lack of access to KRT will create difficult choices for decision makers. Although LMIC should prioritize forms of KRT other than haemodialysis, some haemodialysis capacity will be required 11 , for example, to manage patients with hypercatabolic acute kidney injury or refractory PD-associated peritonitis, which, once available, will inevitably increase the use of this modality.
Health economy-related considerations
The cost of dialysis (especially in-centre or in-hospital dialysis) is high 64 , and the cost per quality-adjusted life-year associated with haemodialysis treatment is often considered to be the threshold value that differentiates whether a particular medical intervention is cost-effective or not 65 . Total dialysis costs across the population will probably continue to rise, owing to increases in life expectancy of the general population and the availability of improved therapeutics for causes of kidney failure such as diabetes mellitus, which have increased the lifespan of these patients and probably will also increase their lifespan on dialysis. KRT absorbs up to 5–7% of total health-care budgets, despite the fact that kidney failure affects only 0.1–0.2% of the general population in most regions 66 . Although societal costs for out-of-centre dialysis (for example, home or self-care haemodialysis, or PD) are in general lower than that of in-centre haemodialysis in many HICs, these options are often underutilized 67 , adding to the rising costs of dialysis.
Reimbursement for haemodialysis correlates with the economic strength of each region 68 , but in part also reflects willingness to pay . In most regions, the correlation curve for PD or reimbursement with respect to gross domestic product projects below that of in-centre haemodialysis, which in part reflects the lower labour costs associated with PD 68 . Unfortunately, little clarity exists with regard to the aggregated cost of single items that are required to produce dialysis equipment for both PD and haemodialysis and the labour costs involved in delivering haemodialysis 69 , which makes it difficult for governments to reimburse the real costs of haemodialysis.
Although increasing reimbursement of home dialysis strategies would seem to be an appropriate strategy to stimulate uptake of these modalities, evidence from regions that offer high reimbursement rates for PD suggests that the success of this strategy is variable 23 , 68 . However, financial incentives may work. In the USA, reimbursement for in-centre and home dialysis (PD or home haemodialysis) has for a long time been identical. The introduction of the expanded prospective payment system in 2011 further enhanced the financial incentives for PD for dialysis providers, which led to a doubling in both the absolute number of patients and the proportion of patients with kidney failure treated with PD 70 , 71 , 72 , 73 .
Although in countries with a low gross domestic product, dialysis consumes less in absolute amounts, it absorbs a higher fraction of the global health budget 68 , likely at the expense of other, potentially more cost-effective interventions, such as prevention or transplantation. Although society carries most of the costs associated with KRT in most HICs, some costs such as co-payment for drugs or consultations are borne by the individual, and these often increase as CKD progresses. In other regions, costs are covered largely or entirely by the patient’s family, leading to premature death when resources are exhausted 74 . In addition, costs are not limited to KRT but also include the costs of medication, hospitalizations and interventions linked to kidney disease or its complications (that is, indirect costs), as well as non-health-care-related costs such as those linked to transportation or loss of productivity.
Dialysis also has an intrinsic economic impact. Patients on dialysis are often unemployed. In the USA, >75% of patients are unemployed at the start of dialysis, compared with <20% in the general population 53 . Unemployment affects purchasing power but also lifestyle, self-image and mental health. Moreover, loss of productivity owing to unemployment and/or the premature death of workers with kidney failure also has economic consequences for society 75 . Therefore, continued efforts to prevent kidney failure and develop KRT strategies that are less time consuming for the patient and allow more flexibility should be an urgent priority. Concomitantly, employers must also provide the resources needed to support employees with kidney failure.
Hence, a pressing need exists to rethink the current economic model of dialysis and the policies that direct the choice of different treatment options. The cost of dialysis (especially that of in-centre haemodialysis) is considerable and will continue to rise as the dialysis population increases. Maintaining the status quo will prevent timely access to optimal treatment for many patients, especially for those living in extreme poverty and with a low level of education and for patients living in LMICs.
Ethical aspects
A 2020 review by a panel of nephrologists and ethicists appointed by three large nephrology societies outlined the main ethical concerns associated with kidney care 76 . With regard to management of kidney failure (Box 1 ), equitable access to appropriate treatment is probably the most important ethical issue and is relevant not only in the context of haemodialysis but also for the other modalities of kidney care (including transplantation, PD and comprehensive conservative care) 76 . Of note, conservative care is not equivalent to the withdrawal of treatment, but rather implies active management excluding KRT.
As mentioned previously, access to such care is limited in many countries 10 , 77 . Inequities in access to dialysis at the individual level are largely dependent on factors such as health literacy, education and socio-economic status, but also on the wealth and organization of the region in which the individual lives. Even when dialysis itself is reimbursed, a lack of individual financial resources can limit access to care. Moreover, elements such as gender, race or ethnicity and citizenship status 78 , 79 can influence an individual’s ability to access dialysis 80 . These factors impose a risk that patients who are most vulnerable are subject to further discrimination. In addition, without necessarily being perceived as such, dialysis delivery may be biased by the financial interests of dialysis providers or nephrologists, for example, by influencing whether a patient receives in-centre versus home dialysis, or resulting in the non-referral of patients on dialysis for transplantation or conservative care 81 , 82 .
A potential reason for the high utilization of in-centre haemodialysis worldwide is a lack of patient awareness regarding the alternatives. When surveyed, a considerable proportion of patients with kidney failure reported that information about options for KRT was inadequate 83 , 84 . Patient education and decision support could be strengthened and its quality benchmarked, with specific attention to low health literacy, which is frequent among patients on dialysis 85 . Inadequate patient education might result from a lack of familiarity with home dialysis (including PD) and candidacy bias among treating physicians and nurses. Appropriate education and training of medical professionals could help to solve this problem. However, the first step to increase uptake of home dialysis modalities is likely policy action undertaken by administrations, but stimulated by advocacy by patients and the nephrology community, as suggested by the higher prevalence of PD at a lower societal cost of regions that already have a PD-first policy in place 68 .
Although the provision of appropriate dialysis at the lowest possible cost to the individual is essential if access is to be improved 86 , approaches that unduly compromise the quality of care should be minimized or avoided. General frameworks to deal with this challenge can be provided by the nephrology community, but trade-offs between cost and quality may be necessary and will require consultation between authorities, medical professionals and patient representatives. Consideration must also be given to whether the societal and individual impact of providing dialysis would be greater than managing other societal health priorities (for example, malaria or tuberculosis) or investing in other sectors to improve health (for example, access to clean drinking water or improving road safety).
The most favourable approach in deciding the most appropriate course of action for an individual is shared decision-making 87 , which provides evidence-based information to patients and families about all available therapeutic options in the context of the local situation. Providing accurate and unbiased information to support such decision-making is especially relevant for conservative care, to avoid the perception that this approach is being recommended to save resources rather than to pursue optimal patient comfort. Properly done, shared decision-making should avoid coercion, manipulation, conflicts of interest and the provision of ‘futile dialysis’ to a patient for whom the harm outweighs the benefits, life expectancy is low or the financial burden is high 88 . However, the views of care providers do not always necessarily align with those of patients and their families, especially in multicultural environments 89 . Medical professionals are often not well prepared for shared decision-making, and thus proper training is essential 90 . Policy action is also required to create the proper ethical consensus and evidence-based frameworks at institutional and government levels 91 to guide decision-making in the context of dialysis care that can be adapted to meet local needs.
Box 1 Main ethical issues in dialysis
Equity in access to long-term dialysis
Inequities in the ability to access kidney replacement therapy exist worldwide; however, if dialysis is available, the ability to transition between different dialysis modalities should be facilitated as much as possible. Specific attention should be paid to the factors that most prominently influence access to dialysis, such as gender, ethnicity, citizenship status and socio-economic status
Impact of financial interests on dialysis delivery
Financial interests of dialysis providers or nephrologists should in no way influence the choice of dialysis modality and/or result in the non-referral of patients for transplantation or conservative care
Cost considerations
Local adaptations are needed to ensure that the costs of dialysis provision are as low as possible without compromising quality of care
The high cost of dialysis means that consideration must be given to whether the benefits obtained by dialysis outweigh those obtained by addressing other health-care priorities, such as malaria or tuberculosis
Shared decision-making
Shared decision-making, involving the patient and their family, is recommended as an approach to allow an informed choice of the most appropriate course to follow
Approaches to shared decision-making must be evidence based and adapted to local circumstances
Futile dialysis should be avoided
Proper training is required to prepare physicians for shared decision-making
Clinical outcomes to measure progress
Over the past six decades, the availability of long-term dialysis has prolonged the lives of millions of people worldwide, often by serving as a bridge to kidney transplantation. Yet, patients on dialysis continue to bear a high burden of disease, both from multimorbidity and owing to the fact that current dialysis modalities only partially replace the function of the native kidney, resulting in continued uraemia and its consequences. Thus, although dialysis prevents death from kidney failure, life expectancy is often poor, hospitalizations (particularly for cardiovascular events and infection) are frequent, symptom burden is high and health-related quality of life is low 22 , 92 , 93 .
Given the multitude of health challenges faced by patients on dialysis, it is necessary to develop a priority list of issues. For much of the past three decades, most of this prioritization was performed by nephrology researchers with the most effort to date focusing on approaches to reducing all-cause mortality and the risk of fatal and non-fatal cardiovascular events. However, despite the many interventions that have been tested, including increasing the dose of dialysis (in the HEMO and ADEMEX trials 94 , 95 ), increasing dialyser flux (in the HEMO trial and MPO trial 94 , 96 ), increasing haemodialysis frequency (for example, the FHN Daily and FHN Nocturnal trials 97 , 98 ), use of haemodiafiltration (the CONTRAST 99 , ESHOL 100 and TURKISH-OL-HDF trials 101 ), increasing the haemoglobin target (for example, the Normal Haematocrit Trial 102 ), use of non-calcium-based phosphate binders (for example, the DCOR trial 103 ), or lowering of the serum cholesterol level (for example, the 4D, AURORA and SHARP trials 104 , 105 , 106 ), none of these or other interventions has clearly reduced all-cause or cardiovascular mortality for patients on dialysis. These disappointments notwithstanding, it is important that the nephrology community perseveres in finding ways to improve patient outcomes.
In the past 5 years, nephrology researchers have increasingly engaged with patients to understand their priorities for meaningful outcomes that should be used to measure progress. The overarching message from this engagement is that although longevity is valued, many patients would prefer to reduce symptom burden and achieve maximal functional and social rehabilitation. This insight highlights the high symptom burden experienced by patients receiving long-term dialysis 92 , 93 , 96 , 107 . These symptoms arise as a consequence of the uraemic syndrome. Some of these symptoms, such as anorexia, nausea, vomiting, shortness of breath and confusion or encephalopathy, improve with dialysis initiation 108 , 109 , 110 , but many other symptoms, such as depression, anxiety and insomnia do not. Moreover, other symptoms, such as post-dialysis fatigue, appear after initiation of haemodialysis.
Of note, many symptoms of uraemic syndrome might relate to the persistence of protein-bound uraemic toxins and small peptides (so-called middle molecules) that are not effectively removed by the current dialysis modalities. The development of methods to improve the removal of those compounds is one promising approach to improving outcomes and quality of life for patients on dialysis, as discussed by other articles in this issue.
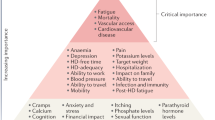
Patients on dialysis report an average of 9–12 symptoms at any given time 92 , 93 , 107 . To determine which of these should be prioritized for intervention, the Kidney Health Initiative used a two-step patient-focused process involving focus groups and an online survey to identify six symptoms that should be prioritized by the research community for intervention. These include three physical symptoms (fatigue, insomnia and cramps) and three mood symptoms (depression, anxiety and frustration) 111 . Parallel to these efforts, the Standardizing Outcomes in Nephrology Group (SONG) workgroup for haemodialysis ( SONG-HD ) has identified several tiers of outcomes that are important to patients, caregivers and health-care providers. Fatigue was identified as one of the four core outcomes, whereas depression, pain and feeling washed out after haemodialysis were identified as middle-tier outcomes 112 , 113 , 114 . Along these same lines, the SONG workgroup for PD ( SONG-PD ) identified the symptoms of fatigue, PD pain and sleep as important middle-tier outcomes 115 , 116 . Despite the importance of these symptoms to patients on dialysis, only a few studies have assessed the efficacy of behavioural and pharmacological treatments on depression 117 , 118 , 119 , 120 , 121 . Even more sobering is the observation that very few, if any, published studies have rigorously tested interventions for fatigue or any of the other symptoms. The nephrology community must now develop standardized and psychometrically robust measures that accurately capture symptoms and outcomes that are important to patients and ensure that these are captured in future clinical trials 122 , 123 .
Approaches to maximizing functional and social rehabilitation are also important to patients with kidney failure. In addition to the above-mentioned symptoms, SONG-HD identified ability to travel, ability to work, dialysis-free time, impact of dialysis on family and/or friends and mobility as important middle-tier outcomes 112 , 113 , 114 . SONG-PD identified life participation as one of five core outcomes, and impact on family and/or friends and mobility as other outcomes that are important to patients 115 , 116 . Given the importance of these outcomes to stakeholders, including patients, it is imperative that nephrology researchers develop tools to enable valid and consistent measurement of these outcomes and identify interventions that favourably modify these outcomes.
Fostering innovation
As described above, the status quo of dialysis care is suboptimal. Residual symptom burden, morbidity and mortality, and economic cost are all unacceptable, which begs the question of what steps are needed to change the established patterns of care. Patients are currently unable to live full and productive lives owing to the emotional and physical toll of dialysis, its intermittent treatment schedule, the dietary and fluid limitations, and their highly restricted mobility during treatment. Current technology requires most patients to travel to a dialysis centre, and current modalities are non-physiological, resulting in ‘washout’, which is defined as extensive fatigue, nausea and other adverse effects, caused by the build-up of uraemic toxins between treatments and the rapid removal of these solutes and fluids over 4-h sessions in the context of haemodialysis. LMICs face additional difficulties in the provision of dialysis owing to infrastructural requirements, the high cost of this treatment, the need for a constant power supply and the requirement for high volumes of purified water. For LMICs, innovations that focus on home-based, low-cost therapies that promote rehabilitation would be especially beneficial.
We contend that initiatives to transform dialysis outcomes for patients require both top-down efforts (for example, those that involve systems changes at the policy, regulatory, macroeconomic and organizational levels) and bottom-up efforts (for example, patient-led and patient-centred advocacy and individual teams of innovators). Top-down efforts are required to support, facilitate and de-risk the work of innovators. Conversely, patient-led advocacy is essential for influencing governmental and organizational policy change. Here, by considering how selected programmes are attempting to transform dialysis outcomes through innovation in support of high-value, high-quality care, we describe how top-down and bottom-up efforts can work synergistically to change the existing ecosystem of dialysis care (Fig. 2 ). The efforts described below are not an exhaustive list; rather, this discussion is intended to provide a representative overview of how the dialysis landscape is changing. Additional articles in this issue describe in more detail some of the bottom-up efforts of innovators to create wearable 124 , portable 125 , more environmentally friendly 126 and more physiological dialysis systems 127 , 128 , priorities from the patients’ perspective 129 , and the role of regulators in supporting innovation in the dialysis space 130 .
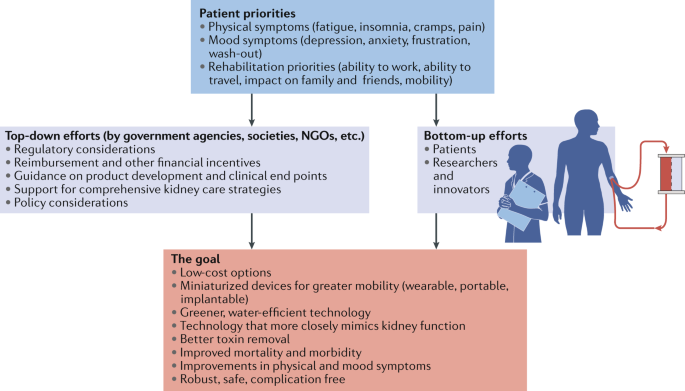
Initiatives to transform dialysis outcomes for patients require both top-down efforts (for example, those that involve systems-level changes at the policy, regulatory, macroeconomic and organizational level) and bottom-up efforts (for example, patient-led and patient-centred advocacy efforts and efforts from individual teams of innovators). Both of these efforts need to be guided by priorities identified by patients. Such an approach, focused on patient-centred innovation, has the potential to result in meaningful innovations that support high-quality, high-value care. NGOs, non-governmental organizations.
The Kidney Health Initiative
In 2012, the American Society of Nephrology (ASN) and the FDA established the KHI as an umbrella organization through which the kidney community can work collaboratively to remove barriers to the development of innovative drugs, devices, biologics and food products, in order to improve outcomes for people living with kidney diseases. To advance its mission, KHI has initiated a number of projects composed of multidisciplinary workgroups. A major accomplishment for the KHI was the establishment of a precompetitive environment to promote innovation while ensuring patient safety.
The KHI is the largest consortium in the kidney community, with over 100 member organizations including patient groups, health professional organizations, dialysis organizations, pharmaceutical and device companies, and government agencies. During the first 7 years of its existence, the KHI has launched and in many cases completed projects that have facilitated the development of new therapeutic options for dialysis patients (Box 2 ), as well as published position papers on topics relevant to innovation in haemodialysis care, including innovations in fluid management 131 and symptom management 132 in patients on haemodialysis, recommendations for clinical trial end points for vascular access 133 , perspectives on pragmatic trials in the haemodialysis population 134 and regulatory considerations for the use of haemodiafiltration 135 .
Box 2 Kidney Heath Initiative Projects that Support Dialysis Innovation
Patient and Family Partnership Council
Since 2015, the Kidney Health Initiative (KHI) Patient and Family Partnership Council (PFPC) has helped KHI stakeholders to engage and network with patients and patient organizations. The PFPC also advises industry and research partners of patient needs and preferences as new products are planned and developed. The PFPC continually emphasizes that innovation will only be successful if built around the needs of people with kidney disease and focused on improving their quality of life.
ESRD Data Standard Project
The aim of this project is to create a harmonized common data standard for kidney failure. The availability of a uniform data standard could accelerate the pace of scientific discovery, facilitate the creation of scientific registries for epidemiological surveillance and allow the development of common metrics for value-based health care.
Building Capacity to Incorporate Patient Preferences into the Development of Innovative Alternatives to kidney replacement therapy (KRT)
This project, which is supported by a 3-year contract with the FDA, is based on the premise that access to scientifically valid patient preference information could positively inform the decisions of industry and regulators as they design and review new devices for individuals with kidney failure. This project will collect patients’ preference information and also address a stated goal of the Advancing American Kidney Health (AAKH) initiative, which instructs the FDA to “develop a new survey to gain insight into patient preferences for new kidney failure treatments” 137 .
Clinical Trial Design to Support Innovative Approaches to KRT
This project is intended to facilitate coordinated efforts between regulators and the nephrology community to streamline the clinical development pathway. The primary objectives of the project are to define terminology for future KRT products (for example, wearable, portable, implantable and artificial kidney) and identify the most appropriate trial designs and end points for a variety of KRT products.
Advancing American Kidney Health
In July 2019, President Donald Trump signed an Executive Order on Advancing American Kidney Health (AAKH) 136 , which promises to fundamentally change the clinical care of kidney disease in general and kidney failure in particular. Components of the AAKH that are relevant to dialysis care include a directive for education and support programmes to promote awareness of kidney disease; a shift in the focus of reimbursement initiatives from in-centre haemodialysis to home therapies, transplantation and upstream CKD care; a system that rewards clinicians and dialysis facilities for providing a range of treatments for kidney failure, with the aim of increasing uptake of home dialysis and transplantation; and incentives for nephrology care teams to focus on reducing costs and improving outcomes by providing longitudinal care of patients with kidney disease.
Finally, and perhaps most radically, the AAKH calls on the US Department of Health and Human Services to support premarket approval of wearable and implantable artificial kidneys and welcomes other strategies to facilitate transformative innovation in dialysis devices. The AAKH directive specifically identifies the KidneyX programme (described below) as the vehicle with which to drive this innovation. The AAKH is the most ambitious US policy initiative ever undertaken to transform the care of patients with advanced kidney disease. Its agenda is still being shaped by the federal governmental agencies, with input from professional societies and other kidney community stakeholders, but this initiative provides a framework and support for transformative innovation in dialysis care.
The KHI Technology Roadmap and KidneyX
The KHI Technology Roadmap for Innovative Approaches to KRT, published in 2019 (ref. 137 ), is aimed at supporting the development of innovative dialysis devices by providing guidance on technical criteria, patient preferences, assessment of patient risk tolerances and regulatory, reimbursement and marketing considerations. Key strengths of the Roadmap include its patient-centred focus and the description of multiple solution pathways for different technologies (for example, portable, wearable and implantable devices that may be purely mechanical, cell-based or hybrid systems), each with appropriate timeline projections.
The KRT Roadmap was designed to be complementary to the Kidney Innovation Accelerator (also known as KidneyX). KidneyX is a public–private partnership between the Department of Health and Human Services and the ASN, and is aimed at accelerating the development of drugs, devices, biologics and other therapies across the spectrum of kidney care. The current major focus of KidneyX is to catalyse the fundamental redesign of dialysis, supported by a series of competitions. Phase I prizes focused on innovations in biomaterials, novel biosensors and safety monitors used for haemodialysis, as well as approaches for improved vascular access and the development of novel technologies that replicate kidney function more precisely than conventional dialysis. Phase II focuses on strategies to build and test prototype solutions or components of solutions that can replicate normal kidney function or improve haemodialysis access. KidneyX has also awarded a series of Patient Innovator Challenge prizes to patients who have proposed innovative solutions to problems emanating from their everyday experiences with kidney disease, including approaches to monitoring blood electrolyte levels and increasing the accessibility of patient education resources.
Dutch Kidney Foundation and Neokidney
The Dutch Kidney Foundation (DKF; or Nierstichting Nederland ) was founded in 1968. It supports research into the causes, prevention and treatment of kidney failure. Furthermore, it works to improve the quality of dialysis treatment and increase the number of kidney transplants. All projects are planned and organized with considerable patient involvement. The DKF also offers financial support to kidney research projects by large Dutch multi-centric consortia. These projects not only promote innovation in the Netherlands but also support trans-national European Union (EU)-supported projects with Dutch participation or leadership, such as Horizon 2020 and Horizon Europe.
Neokidney is a partnership between the DKF and several companies that specialize in miniaturization of dialysis equipment (including dialysis pumps) and sorbent technology for dialysate regeneration. This partnership is aimed at developing a small, portable haemodialysis device that will enable more frequent dialysis sessions, permit more flexibility for patients and improve patient quality of life, as well as reduce health-care costs. The first prototype is currently undergoing preclinical testing and is expected to be tested in humans soon, with the aim of demonstrating proof-of-concept for the first portable haemodialysis machine for daily use, requiring only a limited volume of dialysate. In addition to the development of miniaturization technologies, the partnership is also investigating the use of polymer membranes that permit combined filtration and absorption to achieve more effective haemodialysis 138 .
Nephrologists Transforming Hemodialysis Safety
Nephrologists Transforming Hemodialysis Safety (NTDS) is a collaborative initiative of the ASN and Centers for Disease Control and Prevention (CDC) that is aimed at addressing a specific complication inherent to contemporary dialysis — infection. In 2016, the CDC observed that 10% of dialysis patients in the USA died each year as the result of infections — most of which were preventable. The aim of NTDS is to develop and deploy innovations to achieve zero preventable infections in dialysis facilities across the USA. To reach this goal, NTDS uses a multi-pronged approach. For example, education strategies via publications 139 , 140 , 141 , 142 , 143 and webinars that address various aspects of infection prevention and standards of care, use of social media, development of an interactive chapter for trainees and clinicians, and invited lectures are aimed at ensuring that nephrologists, nurses, dialysis administrators and other professionals understand the risk of dialysis-related infections and evidence-based best working practices.
NTDS also interacts with experts in infection detection, prevention and treatment within federal, state and local health departments who can provide advice and assistance that is independent of the regulatory and potentially punitive arms of health departments. NTDS promotes the appropriate use of these experts in settings where expert advice is needed.
To promote leadership among physicians and nurses, NTDS is sponsoring a leadership academy to ensure that knowledge pertaining to evidence-based best working practices is applied to improve workflows in clinical practice. Effective leadership is a requirement, particularly in complex settings, to ensure that patient safety is prioritized and to motivate staff to use best practices.
NTDS are also collaborating with human factors engineers to study the workflows used in haemodialysis facilities and help to define ways of avoiding errors that lead to infection. As a first step in this process, NTDS and human factors engineers have spent time in various haemodialysis facilities to obtain information about the complex processes of care within those facilities, particularly with regard to the use of haemodialysis catheters and approaches to hand hygiene, injection safety and disinfection. Better understanding of current processes may lead to better workflow design.
Finally, based on lessons learned during the Ebola Crisis of 2014, an NTDS work group has designed processes to anticipate and respond to unexpected health-care crises. At the time of writing this Review, the NTDS team is working with CDC and haemodialysis organizations to anticipate and respond to the COVID-19 epidemic and its effect on dialysis care.
The Affordable Dialysis Prize
As discussed earlier, kidney failure remains a death sentence for many residents of LMICs owing to a lack of access to dialysis. In response to the pressing need for cost-effective dialysis options, the International Society of Nephrology in collaboration with the George Institute for Global Health and the Asian Pacific Society of Nephrology launched the Affordable Dialysis Prize in 2017 with the objective of facilitating the design of a dialysis system that would cost less than US $1,000, and provide treatment for less than $5 a day, yet be as safe and effective as existing dialysis systems. The prize was awarded to an engineer for a system that runs off solar power and includes a miniature distiller for producing pure water from any source via steam distillation. The purified water can then be mixed with electrolytes in empty PD bags to produce cheap, homemade dialysis solutions. This strategy identifies the lack of cheap, high-quality water as a major impediment to dialysis in LMICs and LICs. The system will ideally fit into a small suitcase 144 . This device remains under development with the goal of initiating clinical trials and ultimately commercializing the technology.
Empowered in-centre haemodialysis
For some patients with kidney failure, maintenance in-centre haemodialysis will always be the preferred treatment, and despite incentivizing policy levers, they will not be interested in pursuing home dialysis or kidney transplantation. In-centre self-dialysis (also referred to as empowered haemodialysis) originated in Sweden, when a young engineer named Christian Farman returned to haemodialysis in 2010 after a failed transplant. Farman began negotiating with his nurses to perform his own dialysis treatments with staff supervision and caught the attention of other patients 145 . Eventually, the process of self-dialysis within this centre — whereby coaches in the dialysis unit train people to take over control of their own treatments and health — grew so popular that a new unit was built at the hospital for self-dialysis patients only, with patient input into the design of the unit. Since then, self-care units were installed in several haemodialysis units in Europe and the USA, offering patients the autonomy and flexibility of home haemodialysis within the safety of a controlled environment. This approach to empowering patients has not been widely used to date, but deserves rigorous study and evaluation 146 .
Remote monitoring to support self-care
Telemedicine is defined as the electronic exchange of medical information between sites with the aim of improving a patient’s health. Telehealth encompasses a broader set of services such as the provision of educational content. New technologies have broadened the scope of telemedicine and telehealth applications and services, making these tools more accessible and useful in the care of patients who live remotely or have difficulty visiting a clinic. The range of services that can be delivered by telehealth now includes two-way interactive video, device data programming, asynchronous messaging , sensors for remote monitoring and portals to enable patients to access electronic health records. Although relatively understudied in haemodialysis patients to date, telehealth has the potential to increase the acceptance of home dialysis and improve patient satisfaction, while potentially decreasing costs and improving outcomes.
Telehealth and the remote monitoring of dialysis patients has become more commonplace in the past decade, particularly in Australia, where telehealth is used widely for patients receiving home dialysis. Telemedicine is also considered a support tool for kidney care in disaster situations such as earthquakes where many individuals in remote locations can be affected. Telemedicine has also been used for distance monitoring of patients receiving PD 147 , 148 . In the USA, the Bipartisan Budget Act of 2018 included provisions to expand telehealth coverage to include patients on home dialysis. This legislation allows patients on home dialysis to choose to have their monthly care-provider visits take place via telehealth, without geographic restrictions. The ongoing COVID-19 pandemic has also resulted in an unprecedented and rapid expansion in the use of telemedicine for providing health care in many regions worldwide, including for the care of patients undergoing in-centre haemodialysis. The experience gained during this pandemic has the potential to permanently embed telemedicine in health-care delivery in many health-care systems.
Although telehealth has considerable promise for the care of dialysis patients, the implementation of telehealth in clinical practice can be challenging 149 . Telehealth-guided digital interactions have the potential to improve outcomes through the provision of activities such as individualized patient-centred education, remote communication and data exchange, in-home clinical guidance and monitoring, assessment of prescription and/or treatment efficacy and adherence, real-time modification of treatments and early alerts for problems that require intervention, although all of these interventions need to be rigorously tested 150 .
The European Kidney Health Alliance
The European Kidney Health Alliance (EKHA) is a non-governmental organization based in Brussels, Belgium, which advocates for kidney patients and the nephrology community at relevant bodies of the EU and also at European national organizations. The EKHA represents all of the major stakeholders in kidney care, including physicians, patients, nurses and foundations. The actions of the EKHA are supported by a dedicated group of Members of European Parliament. Of note, according to the treaty of Lisbon 151 , health-care systems are the responsibility of the national authorities of EU countries, which limits the role of the European Commission to one of complementing national policies and fostering cooperation. The EKHA has undertaken several initiatives in the area of kidney care, mainly focusing on measures to decrease the costs of kidney care while maintaining quality of care and access for all appropriate candidates, and to reduce demand for dialysis by promoting efforts to prevent the progression of kidney disease, and encouraging kidney transplantation as the KRT of choice 66 , 152 . In 2021, the EKHA will focus on reimbursement strategies and access to KRT, especially home haemodialysis.
The Nephrology and Public Policy Committee is a similar initiative created by the European Renal Association–European Dialysis and Transplant Association (ERA–EDTA). This committee aims to translate important kidney-related clinical topics into public policy, including the search for novel biomarkers of CKD, improving transition between paediatric and adult nephrology, and improving collaboration between the ERA-EDTA Registry and the guidance body of the ERA-EDTA, European Renal Best Practice 153 .
Beating Kidney Disease
Together with the Dutch Federation for Nephrology and the Dutch Kidney Patients Association, the DKF has initiated a strategic agenda for research and innovation in the Netherlands. This initiative, called Beating Kidney Disease (Nierziekte de Baas) will promote four specific research areas 154 : prevention of kidney failure, including root causes such as other chronic diseases; personalized medicine including genome and big data analyses, and studies of rare diseases; patient-centred outcomes and quality of life, transplantation and home haemodialysis; and regenerative medicine including bio-artificial kidneys. In collaboration with the EKHA, the Beating Kidney Disease initiative will be proposed as a framework for future initiatives at the Directorate General for Health and Food Safety of the European Commission, and the European Commissioner of Health. Similar to European initiatives that have promoted transplantation 152 , 155 , 156 , these efforts will emphasize shifts in policy action to strengthen institutional frameworks, improve education, training and information, optimize registries, and ensure appropriate benchmarking in nephrology.
Conclusions
The past 50 years have seen rapid changes in how and to whom dialysis is provided. From a global perspective, the escalating numbers of patients who require dialysis mean that even current costs are not sustainable, and yet most people who develop kidney failure forego treatment owing to a lack of access, with millions of lives lost every year as a consequence. Also important, the limitations of current dialysis treatment in alleviating patient suffering, morbidity and mortality are now viewed as unacceptable. Consequently, patients, payors, regulators and health-care systems are increasingly demanding improved value, which can only come about through true patient-centred innovation that supports high-quality, high-value care. Substantial efforts are now underway to support requisite transformative changes. These efforts need to be catalysed, promoted and fostered through international collaboration and harmonization to ensure that in the future, people living with kidney failure have more and better treatment options than exist today.
Peitzman, S. J. Chronic dialysis and dialysis doctors in the United States: a nephrologist-historian’s perspective. Semin. Dial. 14 , 200–208 (2001).
Article CAS PubMed Google Scholar
Brescia, M. J., Cimino, J. E., Appel, K. & Hurwich, B. J. Chronic hemodialysis using venipuncture and a surgically created arteriovenous fistula. N. Engl. J. Med. 275 , 1089–1092 (1966).
Blagg, C. R. The early history of dialysis for chronic renal failure in the United States: a view from Seattle. Am. J. Kidney Dis. 49 , 482–496 (2007).
Article PubMed Google Scholar
Scribner, B. H. Ethical problems of using artificial organs to sustain human life. Trans. Am. Soc. Artif. Intern. Organs 10 , 209–212 (1964).
CAS PubMed Google Scholar
Blagg, D. C. R. From Miracle to Mainstream: Creating the World’s First Dialysis Organization (Northwest Kidney Centers, 2017).
Himmelfarb, J., Berns, A., Szczech, L. & Wesson, D. Cost, quality, and value: the changing political economy of dialysis care. J. Am. Soc. Nephrol. 18 , 2021–2027 (2007).
KDIGO. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 3 , 163 (2013).
Google Scholar
Hole, B. et al. Supportive care for end-stage kidney disease: an integral part of kidney services across a range of income settings around the world. Kidney Int. Suppl. 10 , e86–e94 (2020).
Article Google Scholar
Bikbov, B. et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 395 , 709–733 (2020).
Liyanage, T. et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 385 , 1975–1982 (2015).
Tonelli, M. et al. Framework for establishing integrated kidney care programs in low- and middle-income countries. Kidney Int. Suppl. 10 , e19–e23 (2020).
Pecoits-Filho, R. et al. Capturing and monitoring global differences in untreated and treated end-stage kidney disease, kidney replacement therapy modality, and outcomes. Kidney Int. Suppl. 10 , e3–e9 (2020).
Bello A. K. L. et al. Global Kidney Health Atlas: a report by the International Society of Nephrology on the current state of organization and structures for kidney care across the globe. https://www.kidneycareuk.org/documents/52/ISN_Global_kidney_health_atlas.pdf (2017).
White, S. et al. How can we achieve global equity in provision of renal replacement therapy? Bull. World Health Organ. 86 , 229–237 (2008).
Article PubMed PubMed Central Google Scholar
Bello, A. K. et al. Status of care for end stage kidney disease in countries and regions worldwide: international cross sectional survey. BMJ 367 , l5873 (2019).
Luxardo, R. et al. The epidemiology of renal replacement therapy in two different parts of the world: the Latin American Dialysis and Transplant Registry versus the European Renal Association-European Dialysis and Transplant Association Registry. Rev. Panam. Salud Publica 42 , e87 (2018).
United States Renal Data System. Volume 2: ESRD in the United States https://www.usrds.org/2018/download/2018_Volume_2_ESRD_in_the_US.pdf (2018).
Jha, V. et al. The state of nephrology in South Asia. Kidney Int. 95 , 31–37 (2019).
Barsoum, R. S., Khalil, S. S. & Arogundade, F. A. Fifty years of dialysis in Africa: challenges and progress. Am. J. Kidney Dis. 65 , 502–512 (2015).
Bello, A. K. et al. Assessment of Global Kidney Health Care Status. JAMA 317 , 1864–1881 (2017).
Fresenius Medical Care. Annual Report 2018: Care and Live. https://www.freseniusmedicalcare.com/fileadmin/data/com/pdf/Media_Center/Publications/Annual_Reports/FME_Annual-Report_2018.pdf (2018).
United States Renal Data System. US Renal Data System 2019 Annual Data Report: epidemiology of kidney disease in the United States. https://www.usrds.org/2019/view/USRDS_2019_ES_final.pdf (2019).
Liu, F. X. et al. A global overview of the impact of peritoneal dialysis first or favored policies: an opinion. Perit. Dial. Int. 35 , 406–420 (2015).
Article CAS PubMed PubMed Central Google Scholar
Mehrotra, R. et al. Racial and ethnic disparities in use of and outcomes with home dialysis in the United States. J. Am. Soc. Nephrol. 27 , 2123–2134 (2016).
Li, P. K.-T. et al. Changes in the worldwide epidemiology of peritoneal dialysis. Nat. Rev. Nephrol. 13 , 90–103 (2017).
ANZDATA Registry. ANZDATA 42nd Annual Report 2019. https://www.anzdata.org.au/report/anzdata-42nd-annual-report-2019/ (2019).
Kramer, A. et al. The European Renal Association - European Dialysis and Transplant Association (ERA-EDTA) Registry annual report 2015: a summary. Clin. Kidney J. 11 , 108–122 (2018).
Anand, S., Bitton, A. & Gaziano, T. The gap between estimated incidence of end-stage renal disease and use of therapy. PLoS ONE 8 , e72860 (2013).
Modi, G. K. & Jha, V. The incidence of end-stage renal disease in India: a population-based study. Kidney Int. 70 , 2131–2133 (2006).
Robinson, B. M. et al. Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet 388 , 294–306 (2016).
Antlanger, M. et al. Sex differences in kidney replacement therapy initiation and maintenance. Clin. J. Am. Soc. Nephrol. 14 , 1616 (2019).
Chan, K. E. et al. Early outcomes among those initiating chronic dialysis in the United States. Clin. J. Am. Soc. Nephro 6 , 2642–2649 (2011).
Article CAS Google Scholar
Thamer, M. et al. Predicting early death among elderly dialysis patients: development and validation of a risk score to assist shared decision making for dialysis initiation. Am. J. Kidney Dis. 66 , 1024–1032 (2015).
Garcia-Garcia, G. et al. Survival among patients with kidney failure in Jalisco, Mexico. J. Am. Soc. Nephrol. 18 , 1922–1927 (2007).
Foster, B. J., Mitsnefes, M. M., Dahhou, M., Zhang, X. & Laskin, B. L. Changes in excess mortality from end stage renal disease in the United States from 1995 to 2013. Clin. J. Am. Soc. Nephrol. 13 , 91–99 (2018).
Storey, B. C. et al. Declining comorbidity-adjusted mortality rates in English patients receiving maintenance renal replacement therapy. Kidney Int. 93 , 1165–1174 (2018).
Fenton, S. S. et al. Hemodialysis versus peritoneal dialysis: a comparison of adjusted mortality rates. Am. J. Kidney Dis. 30 , 334–342 (1997).
Vonesh, E. F., Snyder, J. J., Foley, R. N. & Collins, A. J. The differential impact of risk factors on mortality in hemodialysis and peritoneal dialysis. Kidney Int. 66 , 2389–2401 (2004).
Mehrotra, R., Chiu, Y. W., Kalantar-Zadeh, K., Bargman, J. & Vonesh, E. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch. Intern. Med. 171 , 110–118 (2011).
Mehrotra, R., Devuyst, O., Davies, S. J. & Johnson, D. W. The current state of peritoneal dialysis. J. Am. Soc. Nephrol. 27 , 3238–3252 (2016).
Mehrotra, R. et al. Chronic peritoneal dialysis in the United States: declining utilization despite improving outcomes. J. Am. Soc. Nephrol. 18 , 2781–2788 (2007).
Modi, Z. J. et al. Risk of cardiovascular disease and mortality in young adults with end-stage renal disease: an analysis of the us renal data system. JAMA Cardiol. 4 , 353–362 (2019).
Wetmore, J. B. et al. Insights from the 2016 peer kidney care initiative report: still a ways to go to improve care for dialysis patients. Am. J. Kidney Dis. 71 , 123–132 (2018).
Skov Dalgaard, L. et al. Risk and prognosis of bloodstream infections among patients on chronic hemodialysis: a population-based cohort study. PLoS One 10 , e0124547 (2015).
Article PubMed PubMed Central CAS Google Scholar
Nelveg-Kristensen, K. E., Laier, G. H. & Heaf, J. G. Risk of death after first-time blood stream infection in incident dialysis patients with specific consideration on vascular access and comorbidity. BMC Infect. Dis. 18 , 688 (2018).
Chaudry, M. S. et al. Risk of infective endocarditis in patients with end stage renal disease. Clin. J. Am. Soc. Nephrol. 12 , 1814–1822 (2017).
Pruthi, R., Steenkamp, R. & Feest, T. UK renal registry 16th annual report: chapter 8 survival and cause of death of UK adult patients on renal replacement therapy in 2012: national and centre-specific analyses. Nephron. Clin. Pract. 125 , 139–169 (2013).
Kucirka, L. M. et al. Association of race and age with survival among patients undergoing dialysis. JAMA 306 , 620–626 (2011).
CAS PubMed PubMed Central Google Scholar
van den Beukel, T. O. et al. The role of psychosocial factors in ethnic differences in survival on dialysis in The Netherlands. Nephrol. Dial. Transpl. 27 , 2472–2479 (2012).
Depner, T. et al. Dialysis dose and the effect of gender and body size on outcome in the HEMO study. Kidney Ing. 65 , 1386–1394 (2004).
Villar, E., Remontet, L., Labeeuw, M. & Ecochard, R. Effect of age, gender, and diabetes on excess death in end-stage renal failure. J. Am. Soc. Nephrol. 18 , 2125–2134 (2007).
Hecking, M. et al. Sex-specific differences in hemodialysis prevalence and practices and the male-to-female mortality rate: the dialysis outcomes and practice patterns study (DOPPS). PLoS Med. 11 , e1001750 (2014).
Erickson, K. F., Zhao, B., Ho, V. & Winkelmayer, W. C. Employment among patients starting dialysis in the United States. Clin. J. Am. Soc. Nephrol. 13 , 265–273 (2018).
Kurella Tamura, M. et al. Functional status of elderly adults before and after initiation of dialysis. N. Eng. J. Med. 361 , 1539–1547 (2009).
Daratha, K. B. et al. Risks of subsequent hospitalization and death in patients with kidney disease. Clin. J. Am. Soc. Nephrol. 7 , 409–416 (2012).
Davison, S. N. & Jhangri, G. S. Impact of pain and symptom burden on the health-related quality of life of hemodialysis patients. J. Pain. Symptom Manage. 39 , 477–485 (2010).
Eneanya, N. D. et al. Longitudinal patterns of health-related quality of life and dialysis modality: a national cohort study. BMC Nephrol. 20 , 7 (2019).
Ju, A. et al. Patient-reported outcome measures for fatigue in patients on hemodialysis: a systematic review. Am. J. Kidney Dis. 71 , 327–343 (2018).
Rhee, E. P. et al. Prevalence and persistence of uremic symptoms in incident dialysis patients. Kidney360 1 , 86–92 (2020).
Kimmel, P. L. & Peterson, R. A. Depression in patients with end-stage renal disease treated with dialysis: has the time to treat arrived? Clin. J. Am. Soc. Nephrol. 1 , 349–352 (2006).
Burnier, M., Pruijm, M., Wuerzner, G. & Santschi, V. Drug adherence in chronic kidney diseases and dialysis. Nephrol. Dial. Transpl. 30 , 39–44 (2015).
Viecelli, A. K. et al. Identifying critically important vascular access outcomes for trials in haemodialysis: an international survey with patients, caregivers and health professionals. Nephrol. Dial. Transpl. 35 , 657–668 (2020).
Ceretta, M. L. et al. Changes in co-morbidity pattern in patients starting renal replacement therapy in Europe-data from the ERA-EDTA registry. Nephrol. Dial. Transpl. 33 , 1794–1804 (2018).
Vanholder, R. et al. Reimbursement of dialysis: a comparison of seven countries. J. Am. Soc. Nephrol. 23 , 1291–1298 (2012).
Winkelmayer, W. C., Weinstein, M. C., Mittleman, M. A., Glynn, R. J. & Pliskin, J. S. Health economic evaluations: the special case of end-stage renal disease treatment. Med. Decis. Mak. 22 , 417–430 (2002).
Vanholder, R. et al. Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat. Rev. Nephrol. 13 , 393–409 (2017).
Klarenbach, S. & Manns, B. Economic evaluation of dialysis therapies. Semin. Nephrol. 29 , 524–532 (2009).
van der Tol, A., Lameire, N., Morton, R. L., Van Biesen, W. & Vanholder, R. An international analysis of dialysis services reimbursement. Clin. J. Am. Soc. Nephrol. 14 , 84–93 (2019).
Beaudry, A. et al. Cost of dialysis therapy by modality in Manitoba. Clin. J. Am. Soc. Nephrol. 13 , 1197–1203 (2018).
Golper, T. A. The possible impact of the US prospective payment system (“bundle”) on the growth of peritoneal dialysis. Perit. Dial. Int. 33 , 596–599 (2013).
Lin, E. et al. Home dialysis in the prospective payment system era. J. Am. Soc. Nephrol. 28 , 2993–3004 (2017).
Shen, J. I. et al. Expanded prospective payment system and use of and outcomes with home dialysis by race and ethnicity in the United States. Clin. J. Am. Soc. Nephrol. 14 , 1200–1212 (2019).
Wang, V. et al. Medicare’s new prospective payment system on facility provision of peritoneal dialysis. Clin. J. Am. Soc. Nephrol. 13 , 1833–1841 (2018).
Swanepoel, C. R., Wearne, N. & Okpechi, I. G. Nephrology in Africa–not yet uhuru. Nat. Rev. Nephrol. 9 , 610–622 (2013).
Wang, V., Vilme, H., Maciejewski, M. L. & Boulware, L. E. The economic burden of chronic kidney disease and end-stage renal disease. Semin. Nephrol. 36 , 319–330 (2016).
Martin, D. E. et al. A call for professional collaboration to address ethical challenges in nephrology. Nat Rev Nephrol. https://doi.org/10.1038/s41581-020-0295-4 (2020).
Harris, D. C. H. et al. Increasing access to integrated ESKD care as part of universal health coverage. Kidney Int. 95 , S1–S33 (2019).
Rodriguez, R. A. Dialysis for undocumented immigrants in the United States. Adv. Chronic Kidney Dis. 22 , 60–65 (2015).
Saunders, M. R., Lee, H., Maene, C., Schuble, T. & Cagney, K. A. Proximity does not equal access: racial disparities in access to high quality dialysis facilities. J. Racial Ethn. Health Disparities 1 , 291–299 (2014).
Shaikh, M. et al. Utilization, costs, and outcomes for patients receiving publicly funded hemodialysis in India. Kidney Int. 94 , 440–445 (2018).
Ladin, K. & Smith, A. K. Active medical management for patients with advanced kidney disease. JAMA Intern. Med. 179 , 313–315 (2019).
Boulware, L. E., Wang, V. & Powe, N. R. Improving access to kidney transplantation: business as usual or new ways of doing business? JAMA 322 , 931–933 (2019).
Van Biesen, W., van der Veer, S. N., Murphey, M., Loblova, O. & Davies, S. Patients’ perceptions of information and education for renal replacement therapy: an independent survey by the European Kidney Patients’ Federation on information and support on renal replacement therapy. PLoS ONE 9 , e103914 (2014).
Mehrotra, R., Marsh, D., Vonesh, E., Peters, V. & Nissenson, A. Patient education and access of ESRD patients to renal replacement therapies beyond in-center hemodialysis. Kidney Int. 68 , 378–390 (2005).
Taylor, D. M. et al. A systematic review of the prevalence and associations of limited health literacy in CKD. Clin. J. Am. Soc. Nephrol. 12 , 1070–1084 (2017).
Vanholder, R., Van Biesen, W. & Lameire, N. Renal replacement therapy: how can we contain the costs? Lancet 383 , 1783–1785 (2014).
Moss, A. H. Revised dialysis clinical practice guideline promotes more informed decision-making. Clin. J. Am. Soc. Nephrol. 5 , 2380–2383 (2010).
Williams, A. W. et al. Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin. J. Am. Soc. Nephrol. 7 , 1664–1672 (2012).
Rinehart, A. Beyond the futility argument: the fair process approach and time-limited trials for managing dialysis conflict. Clin. J. Am. Soc. Nephrol. 8 , 2000–2006 (2013).
Ladin, K. et al. Characterizing approaches to dialysis decision making with older adults: a qualitative study of nephrologists. Clin. J. Am. Soc. Nephrol. 13 , 1188–1196 (2018).
Luyckx, V. A. et al. Developing the ethical framework of end-stage kidney disease care: from practice to policy. Kidney Int. Suppl. 10 , e72–e77 (2020).
Davison, S. N., Jhangri, G. S. & Johnson, J. A. Cross-sectional validity of a modified Edmonton symptom assessment system in dialysis patients: a simple assessment of symptom burden. Kidney Int. 69 , 1621–1625 (2006).
Weisbord, S. D. et al. Renal provider recognition of symptoms in patients on maintenance hemodialysis. Clin. J. Am. Soc. Nephrol. 2 , 960–967 (2007).
Eknoyan, G. et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N. Engl. J. Med. 347 , 2010–2019 (2002).
Paniagua, R. et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J. Am. Soc. Nephrol. 13 , 1307–1320 (2002).
Locatelli, F. et al. Effect of membrane permeability on survival of hemodialysis patients. J. Am. Soc. Nephrol. 20 , 645–654 (2009).
Group, F. H. N. T. et al. In-center hemodialysis six times per week versus three times per week. N. Engl. J. Med. 363 , 2287–2300 (2010).
Rocco, M. V. et al. The effects of frequent nocturnal home hemodialysis: the frequent hemodialysis network nocturnal trial. Kidney Int. 80 , 1080–1091 (2011).
Grooteman, M. P. C. et al. Effect of online hemodiafiltration on all-cause mortality and cardiovascular outcomes. J. Am. Soc. Nephrol. 23 , 1087 (2012).
Farrington, K. & Davenport, A. The ESHOL study: hemodiafiltration improves survival - but how? Kidney Int. 83 , 979–981 (2013).
Maduell, F. et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J. Am. Soc. Nephrol. 24 , 487 (2013).
Besarab, A. et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N. Engl. J. Med. 339 , 584–590 (1998).
Suki, W. N. et al. Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients. Kidney Int. 72 , 1130–1137 (2007).
Wanner, C. et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N. Engl. J. Med. 353 , 238–248 (2005).
Fellstrom, B. C. et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N. Engl. J. Med. 360 , 1395–1407 (2009).
Baigent, C. et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet 377 , 2181–2192 (2011).
Abdel-Kader, K., Unruh, M. L. & Weisbord, S. D. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin. J. Am. Soc. Nephrol. 4 , 1057–1064 (2009).
Mehrotra, R., Berman, N., Alistwani, A. & Kopple, J. D. Improvement of nutritional status after initiation of maintenance hemodialysis. Am. J. Kidney Dis. 40 , 133–142 (2002).
Pupim, L. B. et al. Improvement in nutritional parameters after initiation of chronic hemodialysis. Am. J. Kidney Dis. 40 , 143–151 (2002).
Rivara, M. B. et al. Changes in symptom burden and physical performance with initiation of dialysis in patients with chronic kidney disease. Hemodial. Int. 19 , 147–150 (2015).
Flythe, J. E. et al. Symptom prioritization among adults receiving in-center hemodialysis: a mixed methods study. Clin. J. Am. Soc. Nephrol. 13 , 735–745 (2018).
Tong, A. et al. Establishing core outcome domains in hemodialysis: report of the standardized outcomes in nephrology-hemodialysis (SONG-HD) consensus workshop. Am. J. Kidney Dis. 69 , 97–107 (2017).
Evangelidis, N. et al. Developing a set of core outcomes for trials in hemodialysis: an international Delphi survey. Am. J. Kidney Dis. 70 , 464–475 (2017).
Urquhart-Secord, R. et al. Patient and caregiver priorities for outcomes in hemodialysis: an international nominal group technique study. Am. J. Kidney Dis. 68 , 444–454 (2016).
Manera, K. E. et al. Patient and caregiver priorities for outcomes in peritoneal dialysis: multinational nominal group technique study. Clin. J. Am. Soc. Nephrol. 14 , 74–83 (2019).
Manera, K. E. et al. An international Delphi survey helped develop consensus-based core outcome domains for trials in peritoneal dialysis. Kidney Int. 96 , 699–710 (2019).
Duarte, P. S., Miyazaki, M. C., Blay, S. L. & Sesso, R. Cognitive-behavioral group therapy is an effective treatment for major depression in hemodialysis patients. Kidney Int. 76 , 414–421 (2009).
Taraz, M. et al. Sertraline decreases serum level of interleukin-6 (IL-6) in hemodialysis patients with depression: results of a randomized double-blind, placebo-controlled clinical trial. Int. Immunopharmacol. 17 , 917–923 (2013).
Cukor, D. et al. Psychosocial intervention improves depression, quality of life, and fluid adherence in hemodialysis. J. Am. Soc. Nephrol. 25 , 196–206 (2014).
Friedli, K. et al. Sertraline versus placebo in patients with major depressive disorder undergoing hemodialysis: a randomized, controlled feasibility trial. Clin. J. Am. Soc. Nephrol. 12 , 280–286 (2017).
Mehrotra, R. et al. Comparative efficacy of therapies for treatment of depression for patients undergoing maintenance hemodialysis: a randomized clinical trial. Ann. Intern. Med. 170 , 369–379 (2019).
Mendu, M. L. et al. Measuring quality in kidney care: an evaluation of existing quality metrics and approach to facilitating improvements in care delivery. J. Am. Soc. Nephrol. 31 , 602–614 (2020).
Nair, D. & Wilson, F. P. Patient-reported outcome measures for adults with kidney disease: current measures, ongoing initiatives, and future opportunities for incorporation into patient-centered kidney care. Am. J. Kidney Dis. 74 , 791–802 (2019).
Himmelfarb, J. & Ratner, B. Wearable artificial kidney: problems, progress and prospects. Nat. Rev. Nephrol. in press [doi to be supplied]
Foo, M. W. Y. & Htay, H. Innovations in peritoneal dialysis. Nat. Rev. Nephrol. https://doi.org/10.1038/s41581-020-0283-8 (2020).
Agar, J. W. M. & Barraclough, K. A. Water use in dialysis: environmental considerations. Nat. Rev. Nephrol. https://doi.org/10.1038/s41581-020-0296-3 (2020).
Masereeuw, R. & Verhaar, M. C. Innovations in approaches to remove uraemic toxins. Nat. Rev. Nephrol. https://doi.org/10.1038/s41581-020-0299-0 (2020).
Geremia, I. & Stamatialis, D. Innovations in dialysis membranes for improved kidney replacement therapy. Nat. Rev. Nephrol. https://doi.org/10.1038/s41581-020-0293-6 (2020).
Gedney, N., Sipma, W. & Søndergaard, H. Innovations in dialysis: the user’s perspective. Nat. Rev. Nephrol. https://doi.org/10.1038/s41581-020-0292-7 (2020).
Wieringa, F. P., Sheldon, M. I. & Hidalgo-Simon, A. Regulatory approaches to stimulate innovative renal replacement therapies. Nat. Rev. Nephrol. https://doi.org/10.1038/s41581-020-0275-8 (2020).
Kidney Health Initiatve. Fostering Innovation in Fluid Management. https://khi.asn-online.org/uploads/KHI_InnovationsInFluidManagement.pdf (2019).
Flythe, J. E. et al. Fostering innovation in symptom management among hemodialysis patients. Clin. J. Am. Soc. Nephrol. 14 , 150 (2019).
Shenoy, S. et al. Clinical trial end points for hemodialysis vascular access. Clin. J. Am. Soc. Nephrol. 13 , 490 (2018).
Dember, L. M. et al. Pragmatic trials in maintenance dialysis: perspectives from the kidney health initiative. J. Am. Soc. Nephrol. 27 , 2955 (2016).
Canaud, B., Vienken, J., Ash, S. & Ward, R. A. Hemodiafiltration to address unmet medical needs ESKD patients. Clin. J. Am. Soc. Nephrol. 13 , 1435 (2018).
Trump, D. J. Executive Order on Advancing American Kidney Health. The White House https://www.whitehouse.gov/presidential-actions/executive-order-advancing-american-kidney-health/ (2019).
Kidney Health Initiative. Technology Roadmap for Innovative Approaches to Renal Replacement Therapy. https://www.asn-online.org/g/blast/files/KHI_RRT_Roadmap1.0_FINAL_102318_web.pdf (2018).
Tijink, M. S. L. et al. Mixed matrix membranes: a new asset for blood purification therapies. Blood Purif. 37 , 1–3 (2014).
Vijayan, A. & Boyce, J. M. 100% use of infection control procedures in hemodialysis facilities: call to action. Clin. J. Am. Soc. Nephrol. 13 , 671–673 (2018).
Wong, L. P. Achieving dialysis safety: the critical role of higher-functioning teams. Semin. Dial. 32 , 266–273 (2019).
Kliger, A. S. & Collins, A. J. Long overdue need to reduce infections with hemodialysis. Clin. J. Am. Soc. Nephrol. 12 , 1728–1729 (2017).
Kliger, A. S. Targeting zero infections in dialysis: new devices, yes, but also guidelines, checklists, and a culture of safety. J. Am. Soc. Nephrol. 29 , 1083–1084 (2018).
Collins, A. J. & Kliger, A. S. Urgent: stop preventable infections now. Clin. J. Am. Soc. Nephrol. 13 , 663–665 (2018).
The George Institute. World’s first affordable dialysis machine a finalist in 2017 Eureka Awards. https://www.georgeinstitute.org.au/media-releases/worlds-first-affordable-dialysis-machine-a-finalist-in-2017-eureka-awards (2017).
Institute for Healthcare Improvement. A patient directs his own care. http://www.ihi.org/resources/Pages/ImprovementStories/APatientDirectsHisOwnCareFarmanSelfDialysis.aspx (2020).
Shinkman, R. Is “empowered dialysis” the key to better outcomes? NEJM Catalyst Carryover https://doi.org/10.1056/CAT.18.0232 (2018).
Nayak, K. S., Ronco, C., Karopadi, A. N. & Rosner, M. H. Telemedicine and remote monitoring: supporting the patient on peritoneal dialysis. Perit. Dial. Int. 36 , 362–366 (2016).
Rohatgi, R., Ross, M. J. & Majoni, S. W. Telenephrology: current perspectives and future directions. Kidney Int. 92 , 1328–1333 (2017).
Lew, S. Q. & Sikka, N. Operationalizing telehealth for home dialysis patients in the United States. Am. J. Kidney Dis. 74 , 95–100 (2019).
Bieber, S. D. & Gadegbeku, C. A. A call to action for the kidney community: nephrologists’ perspective on advancing American kidney health. Clin. J. Am. Soc. Nephrol. 14 , 1799–1801 (2019).
Foundation for EU democracy. Consolidated Reader-Friendly Edition of the Treaty on European Union (TEU) and the Treaty on the Functioning of the European Union (TFEU) as amended by the Treaty of Lisbon (2007) Third edition. http://en.euabc.com/upload/books/lisbon-treaty-3edition.pdf (2009).
European Kidney Health Alliance. Thematic Network on Improving Organ Donation and Transplantation in the EU 2019. http://ekha.eu/wp-content/uploads/FINAL_Joint-Statement-of-the-Thematic-Network-on-Organ-Donation-and-Transplantation.pdf (2019).
Massy, Z. A. et al. Nephrology and public policy committee propositions to stimulate research collaboration in adults and children in Europe. Nephrol. Dial. Transpl. 34 , 1469–1480 (2019).
Beating kidney disease. A joint agenda for research and innovation. https://www.nierstichting.nl/media/filer_public/4d/6d/4d6d6b4e-ce56-4a4b-8ba2-f5ac957d0df8/beating_kidney_disease_-_joint_agenda_for_ri_june_2018.pdf (2018).
Matesanz, R., Marazuela, R., Coll, E., Mahillo, B. & Dominguez-Gil, B. About the Opt-Out system, live transplantation, and information to the public on organ donation in Spain… Y ole! Am. J. Transpl. 17 , 1695–1696 (2017).
Zivcic-Cosic, S. et al. Development of the Croatian model of organ donation and transplantation. Croat. Med. J. 54 , 65–70 (2013).
Download references
Author information
Authors and affiliations.
Kidney Research Institute, Seattle, WA, USA
Jonathan Himmelfarb & Rajnish Mehrotra
Division of Nephrology, Department of Medicine, University of Washington, Seattle, WA, USA
Nephrology Section, Department of Internal Medicine and Pediatrics, University Hospital, Ghent, Belgium and European Kidney Health Alliance (EKHA), Brussels, Belgium
Raymond Vanholder
Division of Nephrology, Department of Medicine, University of Calgary, Calgary, Alberta, Canada
Marcello Tonelli
You can also search for this author in PubMed Google Scholar
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Correspondence to Jonathan Himmelfarb .
Ethics declarations
Competing interests.
J.H. declares that The Kidney Research Institute and the Center for Dialysis Innovation at the University of Washington, which he directs, has received gift and grant support from the Northwest Kidney Centers, a not-for-profit dialysis provider. The Center for Dialysis Innovation has also received a Phase I prize from KidneyX, and a grant from the Veterans Administration. J.H. is also a founder and holds equity in AKTIV-X Technologies, Inc. R.V. has consulted for Baxter Healthcare, B. Braun and Neokidney. R.M. has received an honorarium from Baxter Healthcare and serves as a member of the Board of Trustees of the Northwest Kidney Centers. M.T. has received a lecture fee from B. Braun, which was donated to charity.
Additional information
Peer review information.
Nature Reviews Nephrology thanks M. Verhaar, who co-reviewed with M. van Gelder, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Affordable Dialysis Prize: https://www.dialysisprize.org/
Dutch Kidney Foundation: https://www.narcis.nl/organisation/RecordID/ORG1238896/Language/en
ESRD Data Standard Project: https://khi.asn-online.org/projects/project.aspx?ID=78
European Kidney Health Alliance: http://ekha.eu/
Kidney Health Initiative: https://khi.asn-online.org/
KidneyX: https://www.kidneyx.org/
Neokidney: https://www.nextkidney.com/
Nephrologists Transforming Hemodialysis Safety: https://www.asn-online.org/ntds/
Nephrology and Public Policy Committee: https://www.era-edta.org/en/nppc/
Nierstichting Nederland: https://nierstichting.nl/
Patient and Family Partnership Council: https://khi.asn-online.org/pages/?ID=1
SONG-HD: https://songinitiative.org/projects/song-hd/
SONG-PD : https://songinitiative.org/projects/song-pd/
Standardizing Outcomes in Nephrology Group (SONG): https://songinitiative.org/
The total ‘real’ price of a given treatment, including the amount paid by the individual and the amount paid by society.
The ratio of the cost of the intervention compared with a relevant measure of its effect.
The share of treatment cost paid by society; that is, by government or insurers.
Money paid by governments or insurers to health-care providers for money spent on treatment.
The value of everything produced in that country at the prices prevailing in that country, usually expressed as gross domestic product.
The maximum price at or below which a society is prepared to buy a product.
The monetary value of all finished goods and services made within a country during a specific period.
Total amount of money spent by government on health care.
Engineers who design systems, devices, software and tools to fit human capabilities and limitations.
A communication method where the message is placed in a queue, and can be processed at a later time point.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Himmelfarb, J., Vanholder, R., Mehrotra, R. et al. The current and future landscape of dialysis. Nat Rev Nephrol 16 , 573–585 (2020). https://doi.org/10.1038/s41581-020-0315-4
Download citation
Accepted : 19 June 2020
Published : 30 July 2020
Issue Date : October 2020
DOI : https://doi.org/10.1038/s41581-020-0315-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Lipid parameters, adipose tissue distribution and prognosis prediction in chronic kidney disease patients.
- Hui-fen Chen
- Bing-jie Xiao
Lipids in Health and Disease (2024)
Renal rehabilitation learning in Japanese physical therapy schools: a fact-finding study
- Toshiki Kutsuna
- Yuhei Otobe
- Ryota Matsuzawa
Renal Replacement Therapy (2024)
The disruptor of telomeric silencing 1-like (DOT1L) promotes peritoneal fibrosis through the upregulation and activation of protein tyrosine kinases
- Yingfeng Shi
Molecular Biomedicine (2024)
The Intersectoral Coordination Unit for the Sustainable Intensification of Peritoneal Dialysis in Schleswig–Holstein (SKIP-SH) cohort study
- Hauke S. Wülfrath
- Thorben Schrumpf
- Benedikt Kolbrink
BMC Nephrology (2024)
Quality of life and nutritional status in peritoneal dialysis patients: a cross-sectional study from Palestine
- Dania Haddad
- Inad Nawajah
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
Promoting Patient and Nurse Safety in Acute Dialysis Units Through Advocacy and Collaboration
Affiliations.
- 1 Acute Dialysis Nurse, DCH Regional Medical Center, Tuscaloosa, AL.
- 2 American Nephrology Nurses Association (ANNA) National Director (2019-2021).
- 3 member of ANNA's Hazel Taylor Chapter.
- 4 Director, Community Health Initiatives, and Director of Dialysis/Apheresis, Peninsula Regional Medical Center, Delmar, DE.
- 5 Director, International Educators Group, Inc., Los Angeles, CA.
- PMID: 33886246
Hospitals face the increasing challenge of balancing the need to provide quality care with that of being cost-effective. While guidelines and benchmarks for quality care exist in many areas within the hospital, those guidelines can be lacking for the acute (inpatient) dialysis setting. Nephrology nurses working in this setting may provide hemodialysis, peritoneal dialysis, continuous kidney replacement therapy, and apheresis treatments at the patient's bedside and in an acute dialysis unit. This article discusses the need to promote patient and nurse safety in acute dialysis settings through advocacy and collaboration.
Keywords: RN-to-patient ratios; acute dialysis; advocate; certified clinical hemodialysis technicians (CCHTs); nephrology; nurses; quality; safety; staff turnover.
Copyright© by the American Nephrology Nurses Association.
PubMed Disclaimer
Conflict of interest statement
The authors reported no actual or potential conflict of interest in relation to this nursing continuing professional development (NCPD) activity.
Similar articles
- Patient's view of dialysis care: development of a taxonomy and rating of importance of different aspects of care. CHOICE study. Choices for Healthy Outcomes in Caring for ESRD. Rubin HR, Jenckes M, Fink NE, Meyer K, Wu AW, Bass EB, Levin N, Powe NR. Rubin HR, et al. Am J Kidney Dis. 1997 Dec;30(6):793-801. doi: 10.1016/s0272-6386(97)90084-6. Am J Kidney Dis. 1997. PMID: 9398123
- The relationships between nurses' perceptions of the hemodialysis unit work environment and nurse turnover, patient satisfaction, and hospitalizations. Gardner JK, Thomas-Hawkins C, Fogg L, Latham CE. Gardner JK, et al. Nephrol Nurs J. 2007 May-Jun;34(3):271-81; quiz 282. Nephrol Nurs J. 2007. PMID: 17644871
- The patient experience of patient-centered communication with nurses in the hospital setting: a qualitative systematic review protocol. Newell S, Jordan Z. Newell S, et al. JBI Database System Rev Implement Rep. 2015 Jan;13(1):76-87. doi: 10.11124/jbisrir-2015-1072. JBI Database System Rev Implement Rep. 2015. PMID: 26447009
- Peritoneal dialysis for acute renal failure: the safe, effective, and low-cost modality. Ash SR, Bever SL. Ash SR, et al. Adv Ren Replace Ther. 1995 Apr;2(2):160-3. doi: 10.1016/s1073-4449(12)80086-0. Adv Ren Replace Ther. 1995. PMID: 7614347 Review.
- [Acute renal replacement therapy in pediatrics]. Gaillot T, Ozanne B, Bétrémieux P, Tirel O, Ecoffey C. Gaillot T, et al. Ann Fr Anesth Reanim. 2013 Dec;32(12):e231-6. doi: 10.1016/j.annfar.2013.10.020. Epub 2013 Nov 15. Ann Fr Anesth Reanim. 2013. PMID: 24246660 Review. French.
- Analysis of the Influence of Nursing Safety Management on Nursing Quality in Hemodialysis Room. Huang Y, Chen H. Huang Y, et al. Comput Intell Neurosci. 2022 Jul 16;2022:6327425. doi: 10.1155/2022/6327425. eCollection 2022. Comput Intell Neurosci. 2022. Retraction in: Comput Intell Neurosci. 2023 Sep 27;2023:9865457. doi: 10.1155/2023/9865457. PMID: 35880054 Free PMC article. Retracted.
- Search in MeSH
LinkOut - more resources
Full text sources.
- Ovid Technologies, Inc.
- MedlinePlus Health Information
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
Shape ANNA’s Future: Meet the Candidates for President-Elect and Director!

- What is Nephrology Nursing?
- Hear from Nephrology Nurses
- News & Updates
- Membership Types
- Member Directory
- Corporate Opportunities
- Grants & Scholarships
- Ambassador Program
- Member Spotlight
- Free Monthly CE
- Certification Review
- Learning Modules
- Online Library
- Contact Hour Transcripts & Certificates
- CE on Wheels
Nephrology Nursing Journal
- National Symposium
- Nephrology Nursing Summit
- Advocacy Forum
- Nephrology Nurses Week
- Specialty Practice Networks
- Nominate for an Award
- Take Action
- Health Policy
- Legislative Priorities
- Position Statements
- Endorsements & Activities
- Advocacy News
- Volunteer Chapters
- Specialty Practice Networks (SPN)
- Board of Directors
- Roadmaps to Success - Find Your Way!
- Write for the Journal
- Speak at a Conference
- Chat in ANNA Connected
- Connect with your Specialty
- Engage on Social
- ANNA Art Contest
- ANNA Nightingale Tributes
- Member Milestones

The Nephrology Nursing Journal is a refereed clinical and scientific resource that provides current information on a wide variety of subjects to facilitate the practice of professional nephrology nursing. Its purpose is to disseminate information on the latest advances in research, practice, and education to nephrology nurses to positively influence the quality of care they provide.
Information for Advertisers
The Nephrology Nursing Journal is designed to meet the educational and information needs of nephrology nurses in a variety of roles at all levels of practice. It also serves as a source for nonnephrology nurses. Its content expands the knowledge base for nephrology nurses, stimulates professional growth, guides research-based practice, presents new technological developments, and provides a forum for review of critical issues promoting the advancement of nephrology nursing practice.
NNJ has been vetted by the International Academy of Nursing Editors (INANE) and is included in the INANE Nursing Journal Directory.
Access the Electronic Edition
Individual Subscription Options
For subscribers in the USA -
1-year Individual Subscription (USA) - $74
2-year Individual Subscription (USA) - $124
For subscribers outside the USA -
1-year Individual Subscription (Non-USA) - $119
2-year Individual Subscription (Non-USA) - $214
Institutional Subscription Options
1-year Institutional Subscription (USA) - $110
2-year Institutional Subscription (USA) - $173
1-year Institutional Subscription (Non-USA) - $155
2-year Institutional Subscription (Non-USA) - $263
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Strategies to improve dietary, fluid, dialysis or medication adherence in patients with end stage kidney disease on dialysis: A systematic review and meta-analysis of randomized intervention trials
Roles Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Resources, Software, Supervision, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Department of Nephrology, Wollongong Hospital, Wollongong, NSW, Australia
Roles Conceptualization, Formal analysis, Methodology, Resources, Supervision, Validation, Writing – review & editing
Affiliation Centre for Health Research Illawarra Shoalhaven Population (CHRISP), University of Wollongong, Wollongong, NSW, Australia
Roles Conceptualization, Formal analysis, Methodology, Supervision, Validation, Writing – review & editing
Affiliation School of Psychology, University of Wollongong, Wollongong, NSW, Australia
Roles Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Writing – review & editing
Roles Data curation, Formal analysis, Methodology, Project administration, Resources, Writing – review & editing
Roles Conceptualization, Methodology, Resources, Supervision, Writing – review & editing
- Karumathil M. Murali,
- Judy Mullan,
- Steven Roodenrys,
- Hicham C. Hassan,
- Kelly Lambert,
- Maureen Lonergan

- Published: January 29, 2019
- https://doi.org/10.1371/journal.pone.0211479
- Reader Comments
In patients with end stage kidney disease (ESKD) on dialysis, treatment non-adherence is common and results in poor health outcomes. However, the clinical benefits of interventions to improve adherence in dialysis patients are difficult to evaluate since trialled interventions and reported outcomes are highly diverse/ heterogeneous. This review summarizes existing literature on randomized controlled trials (RCTs) evaluating adherence interventions in ESKD patients focusing on the intervention category, outcome efficacy and persistence of benefit beyond the intervention.
We performed electronic database searches in Medline, Embase & Cochrane CENTRAL upto 1 st July 2018 for RCTs evaluating interventions to improve diet, fluid, medication or dialysis adherence in ESKD patients. Study characteristics including category of interventions, outcomes, efficacy and follow-up were assessed. Meta-analysis was used to compute pooled estimates of the effects on the commonest reported outcome measures.
From 1311 citations, we included 36 RCTs (13 cluster-randomized trials), recruiting a total of 3510 dialysis patients (mean age 55.1 ± 5.8 years, males 58.1%). Overall risk of bias was ‘high’ for 24 and of ‘some concern’ for 12 studies. Most interventions (33 trials, 92%) addressed patient related factors, and included educational/cognitive (N = 11), behavioural / counselling (N = 4), psychological/affective (N = 4) interventions or a combination (N = 14) of the above. A majority of (28/36) RCTs showed improvement in some reported outcomes. Surrogate measures like changes in phosphate (N = 19) and inter-dialytic weight gain (N = 15) were the most common reported outcomes and both showed significant improvement in the meta-analysis. Sixteen trials reported follow-up (1–12 months) beyond intervention and the benefits waned or were absent in nine trials within 12 months post-intervention.
Conclusions
Interventions to improve treatment adherence result in modest short-term benefits in surrogate outcome measures in dialysis patients, but significant improvements in trial design and outcome reporting are warranted to identify strategies that would achieve meaningful and sustainable clinical benefits.
Limitations
Poor methodological quality of trials. Frequent use of surrogate outcomes measures. Low certainly of evidence.
Citation: Murali KM, Mullan J, Roodenrys S, Hassan HC, Lambert K, Lonergan M (2019) Strategies to improve dietary, fluid, dialysis or medication adherence in patients with end stage kidney disease on dialysis: A systematic review and meta-analysis of randomized intervention trials. PLoS ONE 14(1): e0211479. https://doi.org/10.1371/journal.pone.0211479
Editor: Wisit Cheungpasitporn, University of Mississippi Medical Center, UNITED STATES
Received: August 16, 2018; Accepted: November 18, 2018; Published: January 29, 2019
Copyright: © 2019 Murali et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: The data is contained in the Supporting Information files.
Funding: The authors received no specific funding for this work.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Adherence to therapy, which is also known as treatment compliance, denotes the extent to which a person’s behaviour of taking medication, following a diet, and / or executing lifestyle changes, corresponds with the recommendations from a health care provider [ 1 ]. Poor adherence to treatment or treatment non-adherence is associated with worse health outcomes, in terms of increased mortality and morbidity [ 2 ]. However, non-adherence is common in patients with chronic diseases and patients with end stage kidney disease (ESKD) who are on dialysis are no exception [ 1 ]. Non-adherence may be intentional or un-intentional and several patient-related, disease-related, and treatment-related factors can contribute to non-adherence in dialysis patients [ 3 ].
Studies evaluating interventions to improve treatment adherence in dialysis patients have broadly addressed four domains of therapy; namely, adherence to recommendations regarding diet, fluid intake, dialysis treatment and medications [ 4 , 5 ]. The lack of standardized methods to measure adherence in these domains, contributes to the reported variations in the rate of non-adherence, and the difficulty of precisely estimating the effectiveness of interventions to improve adherence [ 3 , 5 ]. Methods of measuring adherence vary across studies and include indirect measures, such as self-reported adherence [ 6 , 7 ]; direct measures such as pill counts or electronic medication event monitoring system (MEMS) [ 8 ], and attendance in dialysis sessions [ 9 ]; as well as surrogate measures such as inter-dialytic weight gain [ 10 , 11 ] or biochemical parameters, which include phosphate and potassium levels [ 12 , 13 ].
The interventions to help improve adherence in dialysis patients have also varied between studies. A systematic review of RCTs to improve adherence to dialysis, medication, diet and fluid intake in haemodialysis patients published in 2010, concluded that cognitive behavioural interventions offered the best promise for future studies [ 4 ]. A subsequent review by the same authors [ 5 ] and more recent systematic reviews focusing on specific outcome like inter-dialytic weight gain and phosphate control have included both randomized and non-randomized intervention studies [ 14 , 15 ]. Non-randomized trials make up the majority of adherence intervention studies in dialysis patients [ 5 ], but the lack of random allocation of participants makes them susceptible to selection bias. Several pertinent randomized trials of adherence interventions have been published in the last decade, indicating a keen interest in this research area. In this context, we undertook a systematic review of RCTs in patients with ESKD undergoing dialysis (population), evaluating the effect of interventions to improve dietary, fluid, dialysis or medication adherence (intervention) compared to usual care or alternative strategies (control) on direct, indirect or surrogate measures (outcome) of adherence. Our objectives were to categorize various adherence interventions, examine whether the reported adherence outcomes are clinically meaningful, identify which interventions are effective in improving clinical outcomes and evaluate whether the benefits persist beyond the trialled interventions.
Materials and methods
This systematic review, was structured on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and check-list [ 16 ]. We included randomized trials published as full-text articles in the English language, which evaluated interventions to improve adherence to fluid, diet, medication or dialysis, or a combination of these domains, in ESKD patients undergoing dialysis. The review was registered at PROSPERO, the international prospective register of systematic reviews in February 2018 ( http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018087899 )

Search strategy
Electronic database searches were performed via OvidSP in the Medline, Embase and Cochrane central register of controlled trials for relevant articles using standard search strategies. Medical subject headings or search terms included combinations of ‘dialysis’, ‘renal dialysis’, ‘hemodialysis’, ‘peritoneal dialysis’, ‘patient compliance’, ‘adherence’, ‘medication adherence’, and text word searches using combinations of ‘adheren*’, ‘non-adheren*’ ‘nonadheren*’, ‘complian*’, ‘non-complian*’, ‘noncomplian*’, ‘fluid’, ‘diet’, ‘diet*’, ‘medication’, ‘dialys*’, inter-dialy*’, interdialy*’, ‘haemodialys*’, hemodialys*’, ‘peritoneal dialys*’, and ‘CAPD’ were conducted with searches restricted to ‘English’ and ‘humans’. An example search strategy used for Medline is provided as S1 Table . Search results in the form of titles and abstracts were analyzed by three authors (KM, HH, KL), to identify the studies to be included in the final review, based on the criteria outlined below. Any disagreement was resolved by discussion among all authors. In addition, references in the included articles and other important reviews on the topic were hand-searched to identify articles that might have been missed in the previous searches.
Study selection criteria
Studies that evaluated adult ESKD patients undergoing haemodialysis or peritoneal dialysis were considered. Trials using random allocation of participants to different groups using a parallel group, cluster randomization or randomized crossover design were eligible for inclusion. Studies were included if they trialled at least one intervention, aimed at improving at least one measure of adherence pertaining to one or more domains of ESKD treatment adherence; namely, dietary, fluid, dialysis or medication adherence, as a pre-specified primary or secondary outcome. The reported measure of adherence outcome could have included indirect (e.g. self-reported adherence) or direct (e.g. MEMS-Medication event monitoring system), as well as surrogate measures, which included biochemical parameters (e.g. phosphate level) or inter-dialytic weight gain. For inclusion, studies needed to report the adherence measure before and after the intervention or the change in the adherence measure in response to the intervention. Non-randomized intervention trials and observational studies were excluded as were non-primary research articles (letters to the editor, brief communications and review articles).
Data extraction and synthesis
A standard check-list developed by the authors was used to extract the following data from the included studies: the year of publication, journal, first author’s name, funding source, study design, number of participants in the intervention and control arms, study population characteristics, trialed intervention and control treatments, theoretical model of behaviour underpinning the intervention (if any), primary and secondary outcomes, measures of adherence before and after the intervention or the change in the adherence measures as a result of the intervention, whether adherence was directly measured during the conduct of the study, duration of follow-up, dropout rate, significant secondary outcomes and whether the benefits of intervention persisted on follow-up. If the intervention resulted in significant improvement in the pre-specified direct or indirect adherence efficacy measures (excluding knowledge), the study outcome was considered positive. However, if there was no significant improvement, the study was considered negative, or if there was improvement in some but not all of the pre-specified outcome measures, the study was considered partially positive. One author (KM) extracted the above information into the datasheet, and two authors (HH, KL) verified the accuracy.
A synthesis of the extracted data was then undertaken to group the various interventions under the broad categories of adherence interventions for chronic diseases proposed by the World Health Organization (WHO) [ 1 ], listed as (a) Social and economic interventions, (b) Health system / healthcare team related interventions, (c) Therapy related, (d) Condition or disease related and (e) Patient related interventions.
The patient level interventions were further sub-grouped into the categories of adherence interventions proposed by De Bleser et al [ 17 ]
- Educational/cognitive interventions which provide information or knowledge about disease or treatment to the patient
- Counselling/ behavioural interventions which addressed patient’s behaviour or skill relevant to self-care or empowered them to participate in their care
- Psychologic/affective interventions that appealed to the patient’s feelings and emotions or social support and
- Mixed interventions that involved a combination of the above-mentioned intervention types.
We also undertook meta-analysis to compute a pooled estimate of effect for the most commonly reported outcomes in the included trials.
Quality of included studies
We assessed the risk of bias for the main outcome for each study, rather than applying a ‘quality scale’ to evaluate the methodological quality of the included trials. We opted against the ‘quality scales’ because they tend to combine and assign similar weighting to aspects of study conduct and quality of reporting, which is difficult to justify [ 18 ]. In this review we used the Cochrane risk of bias tool version 2.0 (ROB 2.0) for randomized trials [ 19 ] to assess study quality. Two authors (HH and KL) independently assessed the risk of bias for the five domains of potential bias using the ROB tool. The results were compared to reach a consensus on the risk of bias estimates by consultation and the remaining differences were resolved in discussion with the first author (KM). The overall risk of bias was assessed for each study in a similar fashion.
Statistical methods
Continuous variables were expressed as means ± standard deviation and proportions were expressed as percentages. We compared proportions using Fisher’s exact test. Inter-rater reliability of the risk of bias domains was assessed using Cohen’s kappa statistics. In the meta-analysis, we used the mean difference in the adherence outcome between intervention and control arms as the effect measure, and the random effect option as the analysis model. When the standard deviation of the mean difference was not directly available for use in the meta-analysis from the publication, we contacted the authors seeking this information. However, if it was still unavailable, we computed standard deviation from the confidence intervals or p values cited in the paper, using t-statistics [ 20 ]. In situations where these metrics were not cited in the paper, we imputed the standard deviation from the arithmetic mean [ 20 ] of the standard deviations of the mean difference in the intervention and control arms respectively. Publication bias was evaluated using funnel plots along with the meta-analysis, which was conducted using Review manager software Version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Egger’s test was used to detect the skewness of the funnel plot and objectively assess the publication bias [ 21 ]. The statistical analyses were conducted using Stata version 15.1.
Quality of evidence and ‘Summary of findings’ table
The quality of evidence in this systematic review was rated using the GRADE (Grade of Recommendation, Assessment, Development and Evaluation) approach, which takes into account various factors that can reduce the quality of evidence, such as within-trial risk of bias, inconsistency of results, indirectness of evidence, imprecision and publication bias, as well as factors that improve the quality like large magnitude of effect and dose-response gradient [ 22 ]. We used GRADEpro software to create a summary of findings table for the main outcomes, which provides an overall rating for each outcome and the explanations for grading the evidence [ 23 ].
Search results
Electronic searches in Medline, Embase and Cochrane central register of controlled trials were completed on 1 st July 2018 to identify relevant articles published till that date using search strategies described above. The searches did not limit the range of publication years. The broad electronic database search retrieved 1311 citations out of which 78 adherence intervention trials in dialysis patients were identified, based on the criteria outlined above. Seven trials, which were missed in the database search, were identified by hand search of references from important systematic reviews relevant to the topic. Out of these 85 studies, thirty-six randomized trials which fulfilled the specified inclusion and exclusion criteria were identified for inclusion in the review (for details, please refer to Fig 1 ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0211479.g001
Study characteristics
Out of the 36 studies included in the review, 22 had a parallel group design, while one trial [ 10 ] adopted a randomized crossover design. The remaining studies were cluster randomized trials (for details please refer to Table 1 ).
https://doi.org/10.1371/journal.pone.0211479.t001
The total number of participants in the included studies was 3510, with 1729 in the intervention and 1781 in the control arms. The number of participants in the different trials ranged from 15 to 394 [ 32 , 35 ] with a median of 70 patients. Three trials recruited patients undergoing peritoneal dialysis [ 26 , 32 , 50 ] while the remaining 33 studies recruited haemodialysis patients. The mean age was 55.1years (standard deviation (SD) 5.8years) and male patients constituted a majority (mean 58.1%, SD 12.2%) of the study participants.
Four studies [ 8 , 11 , 24 , 51 ] indicated that they were partly or fully supported by pharmaceutical sponsors, whereas 20 studies were funded by public organisations, including universities. No information on funding source was provided in twelve studies.
Risk of bias
Assessment of included studies using the Cochrane ROB 2.0 tool [ 19 ] with respect to the five domains of potential risk of bias, showed a high inter-rater agreement of 76.7% (between authors HH and KL) based on independently abstracted data (Kappa 0.58, p <0.001), which was further strengthened (inter-rater agreement 95.6%, Kappa 0.92, p <0.001) after consultation. The remaining differences were resolved by discussion between authors. The overall risk of bias in the included trials was judged as ‘high risk’ for 24 studies and ‘some concern’ for the remaining 12 studies. With respect to four out of the five individual risk domains, a majority of the studies were judged to be of ‘some concern’ or ‘high risk’ (for details refer to Fig 2 ). With respect to the ‘risk of bias in measurement of the outcome’, even though blinding of the outcome assessment was not implemented or specified in most of the studies, more than half of the included trials were assessed as ‘low risk’. This was because, the main efficacy measures in these trials were biochemical measurements or changes in body weight, which may not be impacted by the lack of blinding of the outcome assessor. It should be appreciated that these surrogate adherence outcomes are susceptible to measurement error, depending on the test conditions. Changes in the timing of blood sampling in relation to dialysis, length of inter-dialytic interval, or variations in clothing worn by the subject can lead to biased measurement of phosphate levels or body weight. However, such variations will not be modified by outcome assessor blinding and have not been factored into the risk of bias estimates in this review. Additional details of the risk of bias assessment of individual studies are provided in S3 Table .
The data presented for individual risk of bias domains are based on Cochrane ROB 2.0 tool [ 19 ] for randomized trials.
https://doi.org/10.1371/journal.pone.0211479.g002
Interventions
When the article did not describe which aspect of ESKD treatment adherence, -i.e. dietary, fluid-related, dialysis related or medication adherence, was addressed in the study, it was inferred from the nature of the trialled interventions or the reported outcome. For example, reporting of inter-dialytic weight gain as an outcome was interpreted as evaluating adherence to fluid recommendations, while changes in phosphate level as an outcome were interpreted as testing adherence to dietary and medication recommendations. It is acknowledged that these assumptions may not be valid under all circumstances and could lead to misclassification in some cases. Fluid adherence was assessed in nine studies, medication adherence tested in two studies [ 8 , 31 ], while four studies [ 26 , 35 , 36 , 44 ] assessed dietary adherence, and one study each evaluated dialysis adherence [ 40 ] and vascular access cleansing [ 25 ]. The remaining nineteen studies evaluated various combinations of dietary, medication, dialysis and fluid adherence (refer to Table 1 ).
The evaluated intervention was delivered by a variety of health care professionals in the different studies, with dietitians (10 trials) and nurses (9 trials) being the most frequent. Psychologists were the interventionists in seven trials, while a pharmacist [ 43 ] and relaxation therapy professional [ 39 ] delivered the intervention in one study each. The remaining eight studies had multiple staff involved or provided no specific information on the interventionist.
In fifteen studies (42%), theoretical models of behaviour relevant to treatment adherence formed the basis of the trialled intervention. The health belief model [ 27 , 32 , 41 , 45 ] was the most commonly used, while self-efficacy theory [ 7 , 45 , 46 ], social cognitive theory [ 30 , 47 ], self-affirmation theory [ 48 , 49 ], trans-theoretical models (TTM) [ 35 , 37 ], self-regulation theory [ 34 ], King’s theory of goal attainment [ 12 ] and the ABC (Antecedents, Behaviour, Consequences) model relevant to rational emotive therapy [ 33 ] were also invoked. Six out of seven studies, where the interventionist was a psychologist, had a theoretical behavioural underpinning.
Taking into account the five categories of adherence interventions outlined in the WHO report [ 1 ], 33 studies (92%) addressed patient related factors. Health system or health care team related interventions were tested in five studies [ 11 , 31 , 40 , 50 , 51 ], two of which [ 40 , 51 ] also addressed patient related factors. Interventions addressing social-economic factors, therapy related factors or disease or condition related factors were not tested in any of the trials (Refer to Table 1 ).
When we assigned the patient related interventions into the subcategories proposed by De Bleser et al [ 17 ], several studies appeared to align with more than one subcategory. The dominant category was assigned by consensus, with guidance when necessary from the senior psychologist among the authors. Eleven studies evaluated educational or cognitive interventions, four had behavioural or counselling interventions [ 6 , 12 , 27 , 45 ], four had psychological or affective interventions [ 33 , 39 , 48 , 49 ] and fourteen studies had elements of different categories in the trialled intervention (refer to Table 1 ).
Twenty six of the 36 randomized trials (72%) assigned control patients to usual or standard care, including standard health or nutritional education. Wait-listed controls were employed in three studies [ 10 , 32 , 41 ], while the remaining studies used some type of intervention as a comparator to match the active intervention. The comparator included attention control [ 25 ], placebo support and discussion control conditions [ 34 ], provision of matching health information without prior reaffirmation activity [ 48 , 49 ], use of a daily activity monitoring application [ 47 ], and health education which was different from the trialled intervention [ 13 , 28 ]. Four studies [ 7 , 25 , 27 , 35 ] included in this review also had two or more active (partial or alternative) intervention arms in addition to the main intervention and control arms (refer to Table 1 ).
Outcome assessment
The reported outcome data which reflected treatment adherence were diverse and mostly included surrogate measures (refer to Table 1 ). For the only crossover study [ 10 ] in our review, we included the data for the initial phase of randomized comparison between the intervention and control arms, before the crossover. Inter-dialytic weight gain or change in weight or proportion of sessions with satisfactory weight gain was reported as a fluid adherence outcome in 18 studies, while blood pressure (BP) control was assessed in four studies. Biochemical parameters were reported as an adherence outcome in 23 studies, which included combinations of blood levels or change in levels of phosphate (n = 19), calcium (n = 10), calcium phosphate product (n = 7), PTH (n = 7), potassium (n = 5) or albumin (n = 3). Dialysis adherence data, identified as missed or shortened dialysis sessions or changes in biochemical parameters (pre-dialysis BUN, Kt/V), was provided in three studies[ 30 , 40 , 43 ]. Dietary adherence information was provided in two studies [ 9 , 26 ]. Indirect estimates of adherence, as self-reported or otherwise, were given in seven studies, while direct measures of medication adherence using an electronic medication event monitoring system (MEMS) was reported in only one study [ 8 ]. Assessment of knowledge or beliefs about disease or therapy, including medications, or self-efficacy was reported in six studies (refer to Table 1 ).
Outcome efficacy
The trialled intervention was effective in improving the adherence outcome measures in twelve studies, while in sixteen studies the intervention led to mixed results, with improvement in some pre-specified outcome measures but failing to show significant benefit in other adherence outcomes (refer to Table 1 ). For example, in twelve trials which reported two or more biochemical markers as surrogate outcome measures in response to adherence interventions, eight showed improvement in phosphate levels, but five of these studies [ 13 , 24 , 29 , 39 , 51 ] showed no significant improvement in other surrogate adherence outcomes such as calcium x phosphate product, PTH or potassium. The evaluated intervention, did not result in an improvement in any of the adherence outcome measures in eight trials, although two of these studies [ 37 , 45 ] showed improvement in the participant’s knowledge and awareness about the relevant issues, as a result of the intervention (refer to Table 1 ). The outcome efficacy, whether positive or negative, was not significantly associated with the type of interventionist (dietitian or nurses or others) (p = 0.929, Fisher’s exact test), the category of intervention (educational/cognitive or others) (p = 0.388, Fisher’s exact test) or the use of a theoretical model of behaviour (p = 0.694, Fisher’s exact test) in planning the intervention.
Meta-analysis
We computed pooled estimates of the mean differences in phosphate levels and inter-dialytic weight gain in response to the adherence intervention between active and control groups. Though these are only surrogate outcome measures and susceptible to confounding, we chose them for meta-analysis because these were the most commonly reported efficacy measures in the included trials. As shown in Figs 3 and 4 , both phosphate levels and inter-dialytic weight gain significantly improved in response to the adherence intervention, and the level of statistical heterogeneity was moderate (I 2 of 47% and 37% respectively) for the two analyses. As shown in Figs 5 and 6 , the funnel plots did not show significant publication bias in the analysis of nineteen studies reporting a change in phosphate levels (Egger’s test p = 0.901, intercept 0.1, slope -0.33) or fifteen studies reporting a change in inter-dialytic weight gain (Egger’s test p = 0.224, intercept -1.3, slope 0.04) as the adherence outcome. Please refer to S1 and S2 Figs.
The mean difference is measured in mg/dL (to convert mg/dL to mmol/L, multiply by 0.323).
https://doi.org/10.1371/journal.pone.0211479.g003
The mean difference is measured in Kg of body weight.
https://doi.org/10.1371/journal.pone.0211479.g004
https://doi.org/10.1371/journal.pone.0211479.g005
https://doi.org/10.1371/journal.pone.0211479.g006
Duration of intervention and follow-up
The median duration of total follow-up of all the included studies was 129 days, with a minimum of 4 weeks to a maximum of 12 months. The intervention could be as brief as a single educational session lasting less than an hour [ 13 , 24 ] but more typically involved several structured sessions over many weeks. Educational, behavioural and psychological interventions were nearly always conducted on dialysis days in patients on haemodialysis. The duration of follow-up after completion of the intervention varied from 0 days (where the intervention continued until the final outcome assessment) to one year with a median of 42 days. For details of the duration of intervention and total follow-up of individual studies, please refer to Table 1 .
Information on persistence of efficacy of intervention beyond the first outcome assessment was not provided in 20 (56%) studies. Among the remaining 16 studies, the benefits of the trialled intervention had waned or was not detectable by six weeks in one study [ 32 ] three months in four studies [ 8 , 10 , 27 , 43 ], six months in two studies [ 35 , 38 ] and by nine months [ 30 ] and twelve months [ 25 ] in one study each. The impact of the adherence intervention on the outcomes persisted for twelve months after the intervention in two trials [ 48 , 49 ], for six months in two trials [ 34 , 46 ], for three months in two trials [ 41 , 50 ] and for one month in one trial [ 9 ]. For details of the persistence or decline of efficacy of intervention during follow-up of the individual trials, please refer to Table 1 .
Quality of evidence
Confidence in the evidence presented in this review, rated using the GRADE approach was ‘very low’ for the two most commonly reported outcomes. With respect to the factors affecting quality of evidence, we rated down the quality by one level for the domains of risk of bias, inconsistency and imprecision, while we rated down the quality by two levels for indirectness. Publication bias was not detected and did not contribute to the very low certainty of evidence. The summary of findings for these comparisons is given below in Table 2 .
https://doi.org/10.1371/journal.pone.0211479.t002
Our systematic review of randomized intervention trials to improve treatment adherence in dialysis patients shows a moderate, but often partial and short-lasting improvement in pre-specified adherence outcomes. The review also demonstrates a narrow focus on strategies addressing patient related factors, with shortfalls in study design and implementation, some of which are inherent to the non-discrete and heterogeneous nature of adherence behaviour.
Comprehensive adherence interventions should target the patient, the provider and the health care system and address social-economic factors, therapy related, patient related, disease related and health system related factors [ 1 ]. More than 90% of the trials included in our review addressed patient related factors contributing to non-adherence. In dialysis patients, several factors such as access to care, complexity of the treatment regimens, heavy pill burden, lower socio-economic status, poor health literacy and associated comorbidities, such as depression and cognitive impairment, can predispose to non-adherence [ 52 ]. However health care providers often erroneously assume that the patients should be motivated to adhere to the best practice treatment protocol [ 1 ], which might explain why the vast majority of trials have addressed patient related factors. It is important to recognize that the health behaviour of treatment adherence, is the product of diverse but overlapping variables, and comprehensive strategies addressing different issues are required to achieve sustained improvement in adherence.
Change in behaviour such as adherence is a very complex process, parts of which have been conceptualized in various theoretical models of behaviour in over 40% of the included trials. Not surprisingly, trials implemented by psychologists were more likely to invoke such theoretical frameworks including ‘social cognitive theory’, ‘stages of change or trans-theoretical model’, health belief model’ and ‘goal setting theory’. The integrated model of behaviour change or I-change model, which loosely assimilates the above theories and the ‘theory of planned behaviour’, assumes three phases in the process of change, namely awareness, motivation and action [ 53 ]. Information or knowledge about the various aspects of therapy is essential to build awareness, but information by itself is not sufficient to achieve or sustain behaviour change [ 1 ]. Educational or cognitive interventions constituted the sole trialled regimen in one third of the studies in this review, while they were part of the components of the interventions in the majority of studies. Improved knowledge as a result of educational interventions did not translate to a sustained improvement in the measure of adherence, at least in some studies [ 37 , 45 ], confirming that behaviour change requires more than the acquisition of new knowledge.
Many of the RCTs in our review have evaluated varying combinations of educational-cognitive, behavioural-counselling and psychological-affective interventions, with significant overlap between categories. Cognitive behaviour therapy (CBT) refers to a set of intervention strategies aimed at assisting patients in identifying and altering their own dysfunctional cognitions like unhelpful thoughts, beliefs and attitudes, thereby improving their mental well-being and coping behaviour [ 41 ]. In contrast to some short-sighted behavioural modification techniques, a change in belief might enable patients to internalize the cognitive rationale for altering their behaviour [ 27 ]. CBT is a focused psychotherapy aimed at changing the way the individual thinks, feels and behaves, and, unlike traditional psychotherapy it is practical and action-oriented [ 10 ]. The systematic review on interventions to improve adherence in dialysis patients by Matteson et al, [ 4 ] concluded that cognitive behavioural interventions offered the best promise for future trials. In our review, the category of intervention, whether cognitive or behavioural or affective, was not significantly associated with the efficacy of outcome, but the overlap between categories may have affected the reliability of our analysis.
A major issue noted in this review was the frequent use of surrogate outcome measures of adherence. Biochemical and physiological measurements such as phosphate, albumin, Kt/V and inter-dialytic weight gain have been widely used as measures of adherence in dialysis patients [ 4 , 54 ]. Some of these measures can be modified by factors other than adherence, like residual renal function and quality of dialysis [ 5 ]. For example, phosphate levels, which may reflect dietary non-adherence or non-adherence to phosphate binding medications may be affected by inadequate dialysis due to suboptimal vascular access. Marked variations in dialytic phosphate clearance, inconsistency in dietary phosphate absorption, and up to two-fold variations in the efficacy of phosphate binders between individuals, may account for the hyperphosphataemia in dialysis patients, rather than non-adherence to diet and medication [ 55 ]. Similarly, inter-dialytic weight gain may be unreliable as a measure of fluid non-adherence in patients with significant residual urine volume. Failure to address these confounders can lead to misclassification of adherence, and biased efficacy estimates. In this review, self-reported measures of adherence, which are well known to overestimate adherence [ 5 ] were used in one in five studies. However, direct measurement of adherence using ‘pill count’ or the electronic medication event monitoring system (MEMS) which are more robust methods to assess medication adherence [ 1 ], was used in only one out of 36 included trials.
The reporting of a large number of heterogeneous outcomes, many of which are not necessarily patient centred, is not confined to adherence intervention trials but is common in the broader nephrology literature. Initiatives are already underway to identify and implement a set of core outcomes for all trials in haemodialysis (SONG-HD) [ 56 ] and peritoneal dialysis (SONG-PD) [ 57 ] patients, based on the shared priorities of all stakeholders. The widespread adoption of standardized outcome reporting in the future will facilitate meaningful comparisons and pooling of results of dialysis patient trials, including studies evaluating treatment adherence.
A large proportion of the interventions evaluating patient related factors in this review resulted in significant improvement in some of the pre-specified measures of adherence while not impacting on other measures. Since the adherence interventions were not specific for one type of measure over the other, the observed benefits should be interpreted with caution. It is always possible that despite the interventions being outcome non-specific, some of the outcome measures, owing to their biological characteristics, may be more amenable to manipulation than others. We should also remember that when multiple comparisons are made between a study factor and several outcome factors, some of them may return a significant effect due to chance alone.
In a chronic disease like ESKD, it would be reasonable to assume that persistent long-term adherence to therapy would be required to achieve sustained clinical benefits. In our review, the follow-up period for the adherence interventions was relatively short, ranging from four weeks to 12 months. It is conceivable that in patients with ESKD, trials with such short follow-up periods are unlikely to identify any meaningful clinical benefits, other than surrogate markers of adherence which demonstrate a proof of concept about the efficacy of adherence interventions.
Even when a behavioural change in response to an adherence intervention is adopted, relapse of the original behaviour of non-adherence can occur and relapse prevention is an important strategy in achieving long-term adherence [ 37 ]. With brief interventions and short duration of follow-up, there is less opportunity for behavioural reinforcement, with a higher attendant risk of relapse of non-adherence. The majority of the studies (55%) in our review did not report whether adherence persisted beyond the first outcome assessment. In the sixteen studies that provided this information the beneficial effect of adherence intervention waned or was not detectable by 12 months or less in nine studies, while a persistent benefit at 12 months was reported in only two studies. These observations also highlight the importance of having a longer duration of adherence interventions, with appropriate reinforcement strategies, and longer periods of follow-up, to help demonstrate the sustained benefit from such interventions.
Our review has several strengths. It summarizes the research that addresses an important aspect of dialysis patient care, which has the potential to improve patient outcomes. The broad criteria for including trials in this review helped us to cover all important domains of dialysis patient therapy. This has also helped us to identify a significant number of studies including those used in the meta-analysis. Presenting the breadth of categories of adherence interventions and outcomes, in both haemodialysis and peritoneal dialysis patients, based on up to date medical literature, is the main contribution of the current review to the existing research in this field.
However, this review has several limitations. Many of the included trials were small in size, took place in diverse clinical settings and were not of a high methodological quality. The interventions were not homogeneous in nature or intensity. There was a lack of consistency in outcome reporting and follow-up between studies. This made it difficult to effectively compare the interventions or recommend specific strategies to improve treatment adherence in this vulnerable population. The widespread use of surrogate adherence efficacy measures, with the potential for residual confounding, and the relatively short duration of follow-up, made it difficult to judge the true extent of sustainable benefit from these interventions. It is noteworthy that only 8% of the included trials recruited patients on peritoneal dialysis, which could limit the applicability of our review to this population. We included studies which were published as full text articles in English language only and may have missed important trials published in non-English languages. Including such trials may have improved the quality of our review, but this was out of the scope of resources available for this project.
The quality of evidence in this review was rated as ‘very low’ using the GRADE approach. This would imply that the true effect may be substantially different from the effect estimate detected in our review and future high-quality studies may provide different results. The insights from conducting this review enable us to propose, what such a high-quality adherence intervention trial should aim to achieve. The interventions should be well defined and translatable to routine clinical practice. The outcomes should be clinically meaningful, and the study design should specifically address factors other than adherence which can confound the evaluated outcome measures, especially when surrogate measures of adherence efficacy are used. Direct measurement of adherence should be undertaken whenever feasible. The intervention should be of sufficient duration with a plan for periodic reinforcement, which is essential for sustained behaviour change. The follow-up should extend beyond intervention to assess its residual effect and identify the risk of relapse of non-adherence. With an increased interest in the topic in recent years, it is realistic to anticipate that such robust trials will emerge and the results from such trials will enable us to identify effective adherence interventions that would become part of routine clinical care in the future.
In this systematic review we have identified that interventions to improve treatment adherence in dialysis patients are effective in improving surrogate efficacy outcomes, at least in the short term. However, there is considerable scope for improvement in the design and conduct of adherence intervention trials to evaluate whether specific strategies can lead to reliable and lasting benefits with respect to meaningful clinical outcomes in patients with ESKD who are on dialysis.
Supporting information
S1 table. example literature search strategy for medline used in the review..
https://doi.org/10.1371/journal.pone.0211479.s001
S2 Table. Data extraction matrix providing detailed information on study characteristics.
https://doi.org/10.1371/journal.pone.0211479.s002
S3 Table. Risk of bias assessment of individual studies included in the review.
https://doi.org/10.1371/journal.pone.0211479.s003
S1 Fig. Egger’s publication bias plot for trials evaluating change in phosphate levels.
https://doi.org/10.1371/journal.pone.0211479.s004
S2 Fig. Egger’s publication bias plot for trials evaluating change in inter-dialytic weight gain.
https://doi.org/10.1371/journal.pone.0211479.s005
S1 File. PRISMA checklist.
https://doi.org/10.1371/journal.pone.0211479.s006
- 1. Sabate E. Adherence to long-term therapies: Evidence for action.Report of WHO Adherence to Long-term Therapies Project. Geneva, Switzerland: World Health Organization, 2003.
- View Article
- PubMed/NCBI
- Google Scholar
- 5. Matteson ML, Russell C. A systematic review of interventions to increase hemodialysis adherence: 2007–2012. In: Theofilou P, editor. Outcome Assessment in End-Stage Kidney Disease—Measurements and Applications in Clinical Practice: Bentham Science Publishers; 2013. p. 133–66.
- 19. Higgins JPT, Sterne JAC, Savović J, Page MJ, Hróbjartsson A, Boutron I, Reeves B, Eldridge S. A revised tool for assessing risk of bias in randomized trials. Chandler J MJ, Boutron I, Welch V (editors), editor2016.
- 20. Fu R, Vandermeer BW, Shamliyan TA, O’Neil M.E, Yazdi F, Fox SH, et al. Handling Continuous Outcomes in Quantitative Synthesis. (Prepared by the Oregon Evidence-based Practice Center under Contract No. 290-2007-10057-I.) AHRQ Publication No. 13-EHC103-EF. Rockville, MD: Agency for Healthcare Research and Quality. July: 2013.
- 22. Schünemann H BJ, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from https://guidelinedevelopment.org/handbook
- 23. GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University. (developed by Evidence Prime, Inc) Available from https://gradepro.org
- Subscribe to journal Subscribe
- Get new issue alerts Get alerts
Secondary Logo
Journal logo.
Colleague's E-mail is Invalid
Your message has been successfully sent to your colleague.
Save my selection
Improving Outcomes for Patients with Chronic Kidney Disease
Norton, Jenna M. MPH; Newman, Eileen P. MS, RD; Romancito, Gayle RN; Mahooty, Stephanie DNP, MSN; Kuracina, Theresa MS, RD, CDE, LN; Narva, Andrew S. MD, FACP, FASN
Jenna M. Norton is the program manager of the National Kidney and Urologic Science Translation Program at the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health, Bethesda, MD. Andrew S. Narva is the director and Eileen P. Newman is the associate director of the National Kidney Disease Education Program in the Division of Kidney, Urologic, and Hematologic Diseases at the NIDDK. Gayle Romancito is a nurse at the Zuni Comprehensive Community Health Center, Indian Health Service, Zuni, NM. Stephanie Mahooty is an NP at Renal Medicine Associates and Desert Kidney Associates in Albuquerque, NM. Theresa Kuracina is a dietitian at the Albuquerque Indian Health Center, Indian Health Service, Albuquerque, NM. Authors Narva, Newman, and Norton are federal employees of the National Institutes of Health, and Romancito and Kuracina are federal employees of the Indian Health Service. Contact author: Andrew S. Narva, [email protected] . The authors and planners have disclosed no potential conflicts of interest, financial or otherwise.

Coping with chronic kidney disease (CKD) is challenging for many people, since symptoms often don't appear until the disease is advanced and the patient is close to requiring dialysis. This two-part article aims to provide nurses with the basic information necessary to assess and manage patients with CKD. Part 1, which appeared last month, offered an overview of the disease, described identification and etiology, and discussed ways to slow disease progression. Part 2 addresses disease complications and treatment for kidney failure.
The second installment of this two-part article on assessing and managing patients with chronic kidney disease addresses disease complications and treatment for kidney failure.

In Part 1 of this article, which appeared last month, we offered an overview of chronic kidney disease (CKD), described its identification and etiology, and discussed ways to slow disease progression. Here, in part 2, we address disease complications and treatment for kidney failure. As in part 1, the case study of Anna Lowry, a 49-year-old woman with CKD, will be used for illustration, offering nurses specific guidance in helping patients to better understand and manage their CKD. (This case is a composite based on the authors’ experience.)
COMPLICATIONS OF CKD
As kidney function declines, fewer functioning nephrons remain. The complications associated with CKD are complex, and may include anemia, hyperkalemia, hypoalbuminemia, metabolic acidosis, and abnormal mineral metabolism and bone disease. Laboratory work may show multiple metabolic abnormalities. Yet most people don't feel any different until their CKD is quite advanced. As noted in part 1, dietary choices can affect many of the metabolic abnormalities associated with CKD. Thus referral to a registered dietitian who is knowledgeable about CKD may help in managing complications.

There are many symptoms, signs, and laboratory values that must be tracked in patients with progressive CKD. The unique nature of the nurse–patient relationship may allow nurses to pick up on symptoms and signs that the primary care provider might have missed—particularly nonverbal behaviors and cues. It's essential for nurses to alert primary care providers to any subtle changes in a patient's condition, psychosocial issues, patient concerns, abnormal laboratory test results, or trends in laboratory values. (See Case Study: Metabolic Complications .)
Anemia. In the general population, a hemoglobin level of less than 13 g/dL in men and less than 12 g/dL in women indicates anemia. 1, 2 But the optimal target hemoglobin level for people with CKD is currently unknown.
Anemia may occur early in CKD and is generally due to inadequate synthesis of the hormone erythropoietin by the damaged kidneys. The prevalence of anemia increases as the glomerular filtration rate (GFR) declines, affecting nearly 50% of patients with an estimated GFR (eGFR) of less than 30 mL/min/1.73 m 2 . 3 Among patients with advanced CKD, there is evidence that the prevalence of anemia is higher in those with diabetes than in those without diabetes. 4

CKD-associated anemia is generally normochromic and normocytic. 5 That said, identifying and correcting other causes of anemia (such as iron deficiency) is necessary. 5 Assessing iron status in CKD requires a complete blood count and checking iron indices, including serum iron level and total iron-binding capacity (see Table 1 6-9 ). These two results are used to determine the transferrin saturation percentage, which reflects available iron. The serum ferritin level is used to assess stored iron. Optimal target levels of serum iron, total iron-binding capacity, and serum ferritin for people with CKD are unknown. Furthermore, they are affected by inflammation, 10 which is common in CKD, 11 making results more difficult to interpret. The absolute reticulocyte count may also be used to differentiate the cause of anemia or to monitor response to treatment. 8
Ruling out other causes of anemia, including vitamin-deficiency anemia, may also be important. Although megaloblastic anemia is not commonly seen with CKD, some people with diabetes who have taken metformin for years may be vitamin B 12 deficient. Metformin reportedly decreases absorption of this vitamin. 12 Both B 12 and folate levels may be lower than normal with metformin use. 13 Metformin is contraindicated in patients with an eGFR below 30 mL/min/1.73 m 2 . 14
Many other factors may contribute to inadequate iron stores in people with CKD. As their GFR declines, patients may lose interest in high-protein foods. Hepcidin, a hormone that plays a key role in controlling iron levels, regulating iron absorption from the gut, and mobilizing stored iron, accumulates in CKD, 15 and this may result in reduced serum iron levels. Inflammation may also play a significant role in reducing iron absorption. 5
Since iron deficiency is common in CKD, iron status and hemoglobin levels should be checked before the addition of any iron supplements to the regimen. 5 Supplemental iron is available in both oral and IV formulations. Absorption of oral iron supplements may be reduced by the intake of caffeinated beverages, supplemental calcium or calcium-containing antacids, and H 2 -receptor blockers or proton pump inhibitors. 16 Iron supplements may cause unwanted gastrointestinal effects such as heartburn or nausea. Beginning with half the recommended dosage and gradually increasing to the full dosage may help. 16 Patients may also have fewer adverse effects with a different preparation, or by taking iron with food, in divided doses, or along with stool softeners. 16 Injectable erythropoiesis-stimulating agents are used infrequently in treating CKD.
Hyperkalemia. Potassium excretion is regulated by the renin−angiotensin−aldosterone system (RAAS). Perturbations of this system may result in hyperkalemia. Potassium levels tend to increase as GFR declines. 17 Nearly half of patients with an eGFR less than 30 mL/min/1.73 m 2 have serum potassium levels of 4.5 mEq/L or greater. 3 RAAS antagonists may increase the risk of hyperkalemia. Potassium-sparing medications, dietary intake, and transcellular shifts may also affect serum potassium levels.
Several factors can cause potassium to shift between the intracellular and extracellular compartments. Insulin tends to move potassium into the cells; therefore, insulin deficiency can result in hyperkalemia. 18 Metabolic acidosis, characterized by an excess of hydrogen ions in the plasma, may drive potassium out of the cells, as hydrogen ions are buffered intracellularly. 18 As a result, treating both hyperglycemia and acidemia may lower serum potassium. 18 In some patients with CKD, treating acidosis may allow the continued use of RAAS antagonists. 19
Patients with hyperkalemia should be counseled to limit foods that are higher in dietary potassium and to read ingredient lists in order to avoid foods that contain potassium chloride. Beginning in July 2018, food manufacturers will be required to include potassium on the Nutrition Facts label. 20
Hypoalbuminemia occurs in CKD as a result of multiple factors. Both acute and chronic inflammation are associated with reduced albumin synthesis. 21 Loss of albumin in the urine in large quantities is associated with reduced serum albumin levels. Metabolic acidosis, 22 insulin resistance, 23 and a decrease in intake of high-protein foods 24 may also contribute to low serum albumin. One large study of patients on maintenance hemodialysis found that a serum albumin level of greater than 3.8 g/dL was associated with reduced mortality risk, with the lowest such risk seen at levels of 4.4 g/dL or greater. 25 Unfortunately, another study found that only 11% of new dialysis patients had serum albumin levels of 4 g/dL or greater. 26
Metabolic acidosis is usually defined as a serum bicarbonate level of less than 22 mEq/L. The prevalence of decreased serum bicarbonate increases as GFR declines. 27 Nearly a quarter of patients with an eGFR below 30 mL/min/1.73 m 2 have a serum bicarbonate level of 20.5 mEq/L or less. 3 Chronic metabolic acidosis is associated with accelerated muscle degradation, reduced albumin synthesis, exacerbation of metabolic bone disease, impaired glucose tolerance, increased inflammation, and accelerated CKD progression. 28 Animal protein is a source of acid load, while fresh fruits and vegetables are not. Serum bicarbonate levels may increase as dietary protein intake decreases. 29
Interventions to treat chronic metabolic acidosis include an adequate (but not excessive) intake of animal protein and a supplemental base, such as sodium bicarbonate. 28 It's important to note that one 650-mg tablet of sodium bicarbonate has 178 mg of sodium. Reemphasize dietary salt restriction when sodium bicarbonate is prescribed. Some patients may need an added diuretic to help remove the extra sodium. 28
Abnormal mineral metabolism and bone disease. Some CKD patients may have low levels of 25-hydroxyvitamin D, 30 which can trigger complications that affect bone strength and increase the risk of vascular calcification. 31, 32 Serum calcium levels also decrease as a result of low vitamin D levels. 33 Serum phosphorus levels may be within the normal range until CKD is advanced. 33
As eGFR declines, the prevalences of hypocalcemia and hyperphosphatemia increase. 3, 33 Over 19% of patients with an eGFR below 30 mL/min/1.73 m 2 have a serum calcium level of 8.9 mg/dL or less, and nearly 30% have a serum phosphorus level of 4.7 mg/dL or more. 3 The systemic disorders of mineral and bone metabolism associated with CKD are reflected in abnormalities in calcium, phosphorus, parathyroid hormone, and vitamin D metabolism. These derangements result in abnormalities in bone turnover, mineralization, volume, and linear growth or strength. 33 They may be associated with vascular or other soft tissue calcification. 34
Bone disease in people with CKD is complex, and interpreting laboratory test results is difficult. Although observational data support the correction of the metabolic abnormalities, there is limited high-quality evidence to support intervention. Phosphorus restriction may be implemented while trying to maintain adequate protein intake. 30 Phosphorus-binding medications are generally prescribed with meals in order to prevent phosphorus absorption. Low 25-hydroxyvitamin D levels may be treated with ergocalciferol or cholecalciferol, although further trials are needed to confirm the benefits. 31 Elevated parathyroid hormone levels may be managed with vitamin D and phosphorus restriction. 31 Most adults exceed the recommended dietary allowance of 700 mg/day for phosphorus. 35 According to 2011–2012 National Health and Nutrition Examination Survey (NHANES) data, the average phosphorus intake was 1,393 mg/day. 36
Depending on the food source, phosphorus absorption varies. Between 10% and 60% of phosphorus found naturally in protein-rich foods (such as meat, poultry, dairy, nuts, seeds, dried beans, and whole grains) is absorbed. 37 Between 80% and 100% of inorganic phosphorus, which is added to many packaged and processed foods, is absorbed. 37 For example, colas contain phosphoric acid. Sodium phosphates are often added to poultry and pork products to enhance flavor or preserve tenderness. Cereals fortified with calcium may contain calcium phosphate.
Patients should be counseled to check ingredient lists for words containing “phos”—such as phosphorus, sodium phosphate, or pyrophosphate—and to avoid foods that contain such ingredients. Because protein-rich foods tend to contain significant amounts of phosphorus, reducing consumption of such foods may also help in reducing phosphorus intake.
PREPARING FOR KIDNEY FAILURE
Coping with CKD and kidney failure is challenging for many people, since symptoms don't often appear until the disease is advanced and the patient is close to requiring dialysis. Many patients exert great effort in adhering to all treatment recommendations, yet still experience CKD progression. Furthermore, both the disease itself and treatment with dialysis are complicated matters. Patients may have difficulty understanding the implications of changes in their health status. It's not uncommon for patients to experience grief, fear, or depression.
Historically, the health care community has not done a good job in educating CKD patients. An analysis of 1994–1998 and 1999–2000 NHANES data showed that fewer than 20% of patients with both moderately decreased kidney function (an eGFR of 30 to 59 mL/min/1.73 m 2 ) and albuminuria (a urine albumin-to-creatinine ratio greater than 30 mg/g) had ever been told by a physician that they had “weak or failing kidneys.” 38 Such delayed awareness leaves many patients with little time to prepare for kidney failure, leaving them limited options when they face decisions about treatment. As one reflection of inadequate preparation, consider that in 2013 more than 80% of people started hemodialysis with a temporary vascular access (catheter). 39

Many nurses aren't comfortable discussing CKD or dialysis with patients, 40, 41 and may defer such discussions to the nephrologist. This is a missed opportunity, because it's both appropriate and helpful for nurses to initiate these conversations. A new provider may not know the patient well, whereas the nurse in the diabetes or hypertension clinic who has been involved in managing the patient's conditions will have established a trust that can make education more effective. Nurses can help patients to better understand CKD and begin accepting and coping with changes in their health status. (See Case Study: Preparing for Kidney Failure .)
TREATMENT CHOICES FOR KIDNEY FAILURE
There are four options for treating kidney failure. Three involve kidney replacement therapy, including kidney transplantation, peritoneal dialysis, or hemodialysis (either in a dialysis center or at home). The fourth option involves supportive care without transplantation or dialysis.

Transplantation. In general, a kidney transplant is associated with the best quality of life and survival (see Figure 1 42 ). To receive a kidney transplant, a patient must be healthy enough to endure a surgery that can last several hours; have access to a donated kidney, either through a living donor or by being on the waiting list for a deceased donor kidney; and be willing to take antirejection medications daily and to have routine follow-up appointments for the rest of her or his life. Although antirejection medications suppress the immune system, organ rejection remains a possibility. With a functioning transplant, dialysis is not needed, and a near-normal diet can be followed.
Kidney transplantation is a treatment, not a cure. The transplanted kidney will likely work very well for a time—according to recent data trends analyses, 92% of deceased donor and 97% of living donor kidneys continue to work at one year following transplantation, and 47% of deceased donor and 62% of living donor kidneys are still working 10 years later 39 —but eventually it is likely to fail. The outcome improves if the donor and the recipient are ABO blood-type compatible and a match for human leukocyte antigens. Pretransplantation evaluation can take several months and typically includes a comprehensive assessment to check for the presence of any conditions that might place the patient or the transplanted kidney at risk (such as severe coronary artery disease or cancer). Eligibility criteria vary from facility to facility, with some more willing to include patients with a higher body mass index.
Peritoneal dialysis may be a choice for a patient who has no contraindicating abdominal pathology (such as extensive abdominal surgery), wants to do in-home treatment, is willing to perform the treatment daily, and has room to store the necessary supplies. Patients using peritoneal dialysis usually don't require vascular access, but do require minor surgery for abdominal catheter placement.
In peritoneal dialysis, the peritoneal membrane is used as a semipermeable filter, replacing the kidneys. In a peritoneal dialysis exchange, a dialysis solution (the dialysate) flows through the catheter into the abdominal cavity, where it remains for a prescribed period of time known as the dwell time. Through the process of diffusion, waste products move down the concentration gradient from the blood in the peritoneal capillaries into the dialysate. The efficiency of this clearance is determined by the concentration gradient; the size of the solute; and the permeability of the peritoneal membrane, which can vary over time. The dialysate includes an osmotic agent that draws fluid into the peritoneal cavity, removing water and producing some additional clearance by bulk flow. At the end of the dwell time, the solution is drained out through the catheter. The continuous nature of peritoneal dialysis allows the patient to reach equilibrium, avoiding the up-and-down cycles of hemodialysis.
The dialysis prescription must be individualized for each patient. Generally, dextrose is used as the osmotic agent, with the concentration varying based on how much fluid must be removed. The higher the dextrose concentration, the more fluid will be removed—but more dextrose will also be absorbed, elevating blood sugar. Each dialysis exchange is generally two to three liters in volume. The dwell times and the number of exchanges per day vary depending on the patient and the characteristics of the peritoneal membrane.
There are several options for peritoneal dialysis. Continuous ambulatory peritoneal dialysis is performed manually four to five times during the day. Continuous cycling peritoneal dialysis involves the use of a cycler, a small appliance that performs the exchanges automatically. With the cycler, many patients can perform enough exchanges while asleep at night, such that they don't need additional exchanges during the day. Some patients need one or two additional manual exchanges during the day for adequate clearance. Most patients on peritoneal dialysis now use the cycler.
It's important for patients on peritoneal dialysis to restrict their potassium intake. (Such restriction may be greater for patients on hemodialysis.) Amino acids that are lost during the exchanges must be replaced, and the patient's dietary protein needs will be higher. Absorbed dextrose may cause the patient to gain weight. Because peritoneal dialysis is continuous, patients are never in a fasting state, and this has particular implications for those who have diabetes. Glucose levels may be harder to control, but insulin can be added to the dialysis solution. Some patients experience body-image concerns associated with the catheter, and may need psychological and emotional support.

Hemodialysis. In hemodialysis, patients are treated with a hemodialysis machine three or more times a week. A dialyzer serves as the filter, replacing the kidneys. The patient's blood is pumped from the body through tubing, passes through the dialyzer, and is returned to the body. Along the way, blood pressure monitors and airflow detectors ensure the patient's safety. (See Figure 2 .)

The blood enters at the top of the dialyzer and is forced through multiple hollow filaments, each about the size of a human hair. Each filament acts as a semipermeable membrane. As blood passes through the filaments, the dialysis solution flows around the outside of the filaments. It takes less than one second for blood to pass from the top of the dialyzer to the bottom; as it does, waste products diffuse into the dialysate and are carried off, and the blood returns to the body. Diffusion efficiency depends on the size of the solute. Protein-bound substances usually aren't removed; some amino acids, glucose, and water-soluble vitamins are removed. (See Figure 3 .)
In-center hemodialysis may be a choice for a patient who can travel to a dialysis center three times a week for scheduled treatments, prefers that trained staff handle the treatments, doesn't mind venipuncture, and is willing to follow a diet that includes numerous restrictions. Advantages of in-center hemodialysis include the availability of facilities nationwide and the presence of trained staff to do the work. If they so choose, patients can be relatively passive. The staff places the needles, monitors treatment, and maintains the equipment. As with many people with chronic illnesses, people with end-stage kidney disease may become socially isolated, and may enjoy the social setting of the dialysis center. Patients typically spend three to four hours three times a week with the same relatively small group of other patients and providers. Disadvantages include more stringent dietary restrictions, the loss of nutrients during hemodialysis, limited control over the procedure, and the burden of travel to and from the center. Furthermore, hemodialysis patients never reach equilibrium, experiencing instead either a gradual increase in waste products and fluids between treatments or a rapid decrease of these during treatment. These up-and-down cycles may fatigue patients.
Home hemodialysis may be a choice for a patient who wants to perform in-home treatments, has someone to help in doing so, can perform treatments three or more times per week, has room for the machine and supplies, and doesn't mind needlesticks and self-cannulation.
Home hemodialysis is becoming more popular. 39 It requires training and support. As with in-center hemodialysis, home hemodialysis can be done three times per week. But it also permits other options, including daily dialysis for two to three hours, five to six times per week, and nocturnal dialysis for six to eight hours, three or more nights per week. More frequent home dialysis appears to be associated with significant benefits to the patient. 43
Home hemodialysis has a different set of advantages and disadvantages. On the one hand, patients have more control over their schedules, travel isn't required, and the newer machines are smaller and easier to use than the older models were. The diet may be less restrictive, and phosphate binders may be less necessary or not needed. And if treatments are done more frequently, the ups and downs are less severe. On the other hand, home hemodialysis requires that a second person (often a partner or other family member) be present to assist, which may cause stress to the patient, the other person, or both. Either the patient or the person assisting has to insert the needles; and the machine and supplies require space. The patient might have to take time off from work in order to get the initial training, which may not be offered locally. Protein requirements are higher because of protein losses during treatment.
Vascular access . To perform hemodialysis, vascular access must be created. In dialysis, blood usually flows at a rate of about 400 mL/min. Withdrawing blood at that rapid rate from any native peripheral vein would collapse that vein. A blood vessel that can withstand that withdrawal rate without collapse is required.

With permanent vascular access, an artificial connection between an artery and a vein is created, such that some blood is diverted to the vein. This connection may be direct or indirect. An arteriovenous fistula establishes a direct connection, and is the preferred access method, as it's less likely to become infected or to clot (see Figure 4A ). Fistula maturation takes several weeks and involves dilation and thickening of the vein, which occurs as a result of increased blood flow. Once the fistula is mature, access to blood flow for dialysis occurs through a percutaneous needlestick. If a direct connection cannot be created because of small vessel size or another mechanical problem, then an arteriovenous graft is the second option. This connection is made indirectly, using a synthetic tube (see Figure 4B ).
Permanent vascular access is usually established in the nondominant arm. A large IV line (such as a peripherally inserted central catheter) placed in a peripheral vein can destroy that vein for future dialysis use. Patients with CKD should be counseled to protect the blood vessels in both arms by avoiding venipuncture or IV catheter placement above the wrist, if possible. When emergent dialysis must be performed, temporary vascular access may be established using a central vein, usually by placing a catheter in the internal jugular vein in the neck. However, this is only a temporary solution. Catheters are associated with inadequate dialysis, increased infection rates, increased clotting, and inflammation. 44
Supportive treatment without transplantation or dialysis. Opting for neither transplantation nor dialysis may be right for patients who feel that such treatments won't improve their health; feel they've done what they wanted to do in life; and, ideally, have family and friends who support their decision. Supportive treatment involves active medical management in which many complications can be treated. Medications for CKD are usually continued and can be adjusted. However, with supportive treatment there is no medical intervention aimed at replacing lost kidney function. Without clearance of uremic toxins, the patient will eventually become uremic.
It's essential to provide comfort and palliative care to these patients. Patients who choose supportive therapy need to understand that without kidney replacement therapy, they will eventually die from uremia; it's important that their family members understand this also. These facts must be presented in a manner that doesn't question the patient's decision, yet ensures that the decision is an informed one.
For additional information on caring for patients with CKD, visit the Web site of the National Kidney Disease Education Program ( http://bit.ly/2gaGy4w ).
For eight additional continuing nursing education activities on topics related to kidney disease, go to www.nursingcenter.com/ce .
chronic kidney disease; collaborative care; end-stage kidney failure; end-stage renal failure; interdisciplinary care; kidney disease
- + Favorites
- View in Gallery
Readers Of this Article Also Read
Ce: improving outcomes for patients with chronic kidney disease: part 1, using evidence-based practice to prevent hospital-acquired pressure ulcers and..., nursing in pneumonia, finerenone added to treatment guidelines for type 2 diabetes and chronic kidney ..., complications of chronic kidney disease: a close look at renal osteodystrophy,....

- Featured Feed
- Collections
- Become A Contributor
- Account Settings
- My Public Profile
- Feed Subscriptions
- Change My Institution
Use the journals feature with a free QxMD account.
Use Read by QxMD to access full text via your institution or open access sources.
Read also provides personalized recommendations to keep you up to date in your field.
Existing User
New to Read
Sign up for a free QxMD account to keep track of pertinent research with access to hundreds of Journal Publications.
Nephrology Nursing Journal : Journal of the American Nephrology Nurses' Association
All material on this website is protected by copyright, Copyright © 1994-2024 1994-{new Date().getFullYear()} by WebMD LLC. By using this service, you agree to our terms of use and privacy policy .
Save your favorite articles in one place with a free QxMD account.
Search tips.
Use Boolean operators: AND/OR
diabetic AND foot diabetes OR diabetic
Exclude a word using the 'minus' sign
Virchow -triad
Use Parentheses
water AND (cup OR glass)
Add an asterisk (*) at end of a word to include word stems
Neuro* will search for Neurology, Neuroscientist, Neurological, and so on
Use quotes to search for an exact phrase
"primary prevention of cancer" (heart or cardiac or cardio*) AND arrest -"American Heart Association"
We want to hear from doctors like you!
Take a second to answer a survey question.

Ethiopian Journal of Health Sciences Journal / Ethiopian Journal of Health Sciences / Vol. 34 No. 5 (2024) / Articles (function() { function async_load(){ var s = document.createElement('script'); s.type = 'text/javascript'; s.async = true; var theUrl = 'https://www.journalquality.info/journalquality/ratings/2409-www-ajol-info-ejhs'; s.src = theUrl + ( theUrl.indexOf("?") >= 0 ? "&" : "?") + 'ref=' + encodeURIComponent(window.location.href); var embedder = document.getElementById('jpps-embedder-ajol-ejhs'); embedder.parentNode.insertBefore(s, embedder); } if (window.attachEvent) window.attachEvent('onload', async_load); else window.addEventListener('load', async_load, false); })();
Article sidebar.

Article Details
Main article content, integrated dialysis nursing intervention for ameliorating fatigue in hemodialysis patients, yogalakshmi s., sasikala d., santosh varughese, vasanthakumari sundararajan.
BACKGROUND: Fatigue is a pervasive and debilitating symptom among hemodialysis patients, severely impacting their quality of life and ability to participate in social activities. Dialysis nurses are pivotal in alleviating these effects through physical exercise. This study aims to evaluate the effectiveness of an integrated dialysis nursing intervention in reducing fatigue among hemodialysis patients.
METHODS: A quasi-experimental time series design was employed, involving 295 hemodialysis patients (148 in the experimental group and 147 in the control group) selected through consecutive sampling from two dialysis units in Chennai. Baseline fatigue was assessed in both groups. The experimental group received the integrated dialysis nursing intervention, including 15-minute sessions of aerobic exercises three times a week for eight weeks. The control group continued with routine care. Fatigue levels were reassessed at the end of the fourth and eighth weeks. Data were analyzed using SPSS version 20.
RESULTS: The study revealed a significant reduction in fatigue scores in the experimental group compared to the control group, with p < 0.001 in post-test I and II. The experimental group showed greater improvement than the control group, with p < 0.05.
Conclusions: The integrated dialysis nursing intervention significantly reduced fatigue in hemodialysis patients. Incorporating this approach into routine intradialytic care can enhance fatigue management and improve patients' quality of life.
AJOL is a Non Profit Organisation that cannot function without donations. AJOL and the millions of African and international researchers who rely on our free services are deeply grateful for your contribution. AJOL is annually audited and was also independently assessed in 2019 by E&Y.
Your donation is guaranteed to directly contribute to Africans sharing their research output with a global readership.
- For annual AJOL Supporter contributions, please view our Supporters page.
Journal Identifiers

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

Dialysis and Fatigue: Implications for Nurses – A Case Study Analysis
Ann horigan.
Duke University School of Nursing, Harrisonburg, VA
Judith Rocchiccioli
James Madison University, Harrisonburg, VA
Donna Trimm
Fatigue is one of the most common symptoms experienced by patients receiving dialysis. When patients with chronic kidney disease (CKD) and end-stage renal disease are admitted to acute care settings, they require management of their often profound fatigue. CKD, renal pathology, and renal fatigue are examined in relation to a case study.
Caring for patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD) challenges all health care providers. While a great deal of the maintenance care for patients with ESRD occurs at hemodialysis centers in the community, admission of affected patients to the hospital requires nurses to demonstrate knowledge of renal disease and renal pathology, and expertise in the identification and management of the debilitating fatigue that often impacts patients’ quality of life. Research suggests fatigue is one of the most common symptoms experienced by patients receiving dialysis ( Jablonski, 2007 ; Weisbord et al., 2005 ). Prevalence of fatigue ranges from 60% to 97% ( Murtaugh, Addington-Hall, & Higginson, 2007 ; Weisbord et al., 2005 ). Assessment and management of fatigue thus are important in improving clinical outcomes and quality of life for patients receiving dialysis.
Several factors may be associated with fatigue in patients receiving dialysis. These include depression ( Leinau, Murphy, Bradley, & Fried, 2009 ; Liu, 2006 ), female sex ( Liu, 2006 ; O’Sullivan & McCarthy, 2007 ), and anemia ( Williams, Crane, & Kring, 2007 ). While the cause of renal fatigue remains unclear, it is clearly problematic. An understanding of how patients receiving dialysis describe and experience fatigue, the similarities and differences in fatigue experienced by patients receiving hemodialysis and peritoneal dialysis, and knowledge of the cultural differences in the experience of fatigue are critical in order to assess it accurately and intervene appropriately. Chronic kidney disease, renal pathology, and renal fatigue are discussed and related to the case of Mary R.
Overview of Renal Pathology and Renal Disease
Incidence and prevalence of end-stage renal disease.
End-stage renal disease, the last stage of chronic kidney disease, is a common chronic illness that is increasing in incidence and prevalence. The incidence of ESRD in 2006 was 360 per million, an increase of 2.1% since 2005. Patients receiving dialysis now live longer, with the mortality rate decreased by 10%. Additionally, the rate of kidney transplantation has not kept pace with the incidence of ESRD, and increasing numbers of patients with failed transplants are returning to hemodialysis. In 2006, the number of new patients receiving dialysis or transplant in the United States exceeded 100,000; over 350,000 patients were receiving dialysis ( National Institute of Diabetes and Digestive and Kidney Disease [NIDD KD], 2008 ). As the number of affected patients continues to grow and medical advances allow patients to lead longer lives, symptom management becomes an important part of care for patients receiving dialysis.
Overview of Chronic Kidney Disease
The current National Kidney Foundation (NKF) guidelines define chronic kidney disease as irreversible kidney damage or decreased kidney function for at least 3 months ( Castner, 2010 ; Murphy, Jenkins, Chamney, McCann, & Sedgewick, 2008 ; NKF, 2002 ), which ultimately affects all kidney functions. The nephrons, the functional unit in the kidneys, lose their ability to filter wastes and extra fluid, creating fluid and electrolyte imbalances. Loss of kidney function occurs slowly, with the kidneys initially adapting well to the underlying causes of kidney damage ( Castner, 2010 ). Symptoms of kidney failure, such as alterations in salt and fluid balance, protein-urea, and anemia, do not appear until renal function decreases significantly ( Molzahn & Butera, 2006 ).
The severity of kidney disease is determined by the individual’s glomerular filtration rate (GFR), which is the best measure of the overall health and function of the kidneys ( Kinzner & Hain, 2007 ). The glomerulus is a collection of capillaries in the nephron, and the glomerular membranes act as the filtering mechanism for wastes and fluid. The rate at which the glomerulus filters wastes and fluid is an indication of its health. A GFR of 90 mL/minute per 1.73 m 2 or higher is considered normal. A decreasing GFR signifies increasing kidney damage. A GFR less than 60 mL/minute per 1.73 m 2 indicates a loss of approximately half the kidney’s normal function in an adult. As the GFR continues to decrease in kidney failure, occurrence of complications related to kidney disease, such as anemia, bone disease, and malnutrition, increases ( Castner, 2010 ; NKF, 2002 ).
Five stages of chronic kidney disease have been identified ( NKF, 2002 ) (see Table 1 ). A healthy kidney is able to differentiate between protein and wastes when filtering the blood. A damaged kidney is unable to do this, and thus protein is excreted inappropriately along with the wastes in the urine. The early stages of CKD are characterized by protein-urea and decreasing GFR. Many complications, such as anemia and bone disease, become evident as CKD progresses ( NKF, 2011 ). During the later stages of CKD, continued management of complications as well as preparation for renal replacement therapy (dialysis or transplantation) are important. When the kidneys have failed completely and irreversibly, renal replacement therapy is necessary to sustain life. At this point, most patients experience symptoms of uremia ( NKF, 2002 ), including decreased appetite, malnutrition, drowsiness, shortness of breath, and palpitations ( Murtaugh et al., 2007 ).
Stages of Chronic Kidney Disease
| Stage | Defining Characteristics |
|---|---|
| Stage 1 CKD | GFR normal (90 mL/min) |
| Proteinurea | |
| Stage 2 CKD | GFR 60–89 mL/min |
| Proteinurea | |
| Stage 3 CKD | GFR 30–59 mL/min |
| Proteinurea | |
| Anemia and bone disease may become evident. | |
| Stage 4 CKD | GFR 15–29 mL/min |
| Management of decreased kidney function | |
| Stage 5 CKD (ESRD) | Preparation for dialysis |
| GFR <15 mL/min | |
| Dialysis initiated | |
| Symptoms of uremia | |
GFR = glomerular filtration rate
Adapted from National Kidney Foundation, 2002 .
Common Causes of Chronic Kidney Disease
One of the most common causes of CKD is diabetes. Other causes include hypertension and glomerulonephritis ( NIDDKD, 2010 ). When a patient has diabetes, the glomerulus is damaged. The exact mechanism of damage is unknown, but the most likely cause is denaturization of proteins caused by high blood glucose. This leads to a thickening of the membranes of the capillaries in the glomerulus that eventually results in scarring and stenosis of the capillaries ( Bakris, 2011 ; Redmond & McClelland, 2006 ). The stenosis of glomerular capillaries causes an inability to filter wastes properly, and contributes to fluid and electrolyte imbalances in the body.
Another common cause of CKD is untreated hypertension ( NIDDKD, 2010 ). Normal regulatory processes in the kidney are compromised when the systemic blood pressure remains elevated over a long period of time, as in untreated or inadequately treated hypertension. First, untreated systemic hypertension causes damage to the systemic blood vessels by causing them to thicken and strengthen to withstand the increased pressure. These vessels become permanently narrowed due to thickening. The arteries that feed the kidneys respond to high systemic pressures by constricting, thus decreasing the amount of blood circulating through the kidneys. The kidneys in turn interpret this as a deficit in blood flow. In response, renin and aldosterone secretion are stimulated, leading to sodium and water retention in order to increase blood volume. This response only perpetuates the systemic hypertension ( Hill, 2008 ). Over time, as the kidneys lack appropriate perfusion due to arterial constriction, nephron damage results and efficient filtration of water and wastes is lost.
Glomerulonephritis is another common cause of kidney failure ( NIDDKD, 2010 ). Cellular, immunologic, and inflammatory factors that damage the glomerulus (capillaries in the nephron) often are triggered by changes in tissue or infections ( Redmond & McClelland, 2006 ). Glomerulonephritis can range from mild to severe, and damage to the glomeruli differs from person to person. Nephrons are destroyed in a predictable pattern that begins with cellular infiltration and exudative reactions. Damage then progresses to macrophage infiltration with differing levels of parenchymal cell proliferation. These changes eventually lead to sclerotic structural changes in the nephron, damaging it and resulting in an inability to function properly ( McCance & Huether, 2010 ).
Complications of Chronic Kidney Disease
As kidney function declines, multiple body systems are affected detrimentally by the accumulation of toxins in the blood (uremia) ( Alper, Shenava, & Young, 2010 ). These symptoms usually begin to appear in stage 3 kidney disease and worsen as kidney function declines to ESRD. The kidneys lose the ability to excrete electrolytes, causing serum electrolyte imbalances. As serum phosphate increases, the additional phosphate binds with calcium and a decrease results in serum calcium levels. The body responds by pulling calcium from the bone to maintain appropriate serum calcium ( McCarley & Arjomand, 2008 ). Bone demineralization, pain, and spontaneous fractures thus can occur as kidney disease progresses ( NIDDKD, 2009a ).
Serum potassium continues to rise, even to critical levels, as CKD continues. Hyperkalemia can result in muscle weakness, increased neuromuscular irritability manifested as tingling in the fingers and lips, restlessness, stomach cramping, and diarrhea. At critically high levels, potassium can cause changes in the EKG complex, often in the form of bradyarythmias. Conduction is slowed through the heart muscle, resulting in prolonged PR intervals and a widening QRS complex, often resulting in ventricular fibrillation or cardiac arrest ( Putcha & Allon, 2007 ).
Erythropoietin secretion is controlled by the kidneys and is compromised as kidney failure progresses. Red blood cell production in the bone marrow then decreases, resulting in anemia. Additionally, the red cells that are produced have a shortened life span due to the build-up of toxins in the blood ( Alper et al., 2010 ).
Another complication of CKD is impaired creatinine and urea clearance ( Broscious & Castagnola, 2006 ). Creatinine is released constantly from the muscle. As the GFR decreases, the serum creatinine values increase. High serum creatinine is an indicator of kidney dysfunction ( NIDDKD, 2009b ). Urea, the end product of protein metabolism, also increases as the kidneys fail. Retention of urea can cause loss of appetite, nausea, vomiting, and pancreatitis ( McCance & Huether, 2010 ).
Dialysis in Chronic Kidney Disease
Complications of CKD worsen as renal failure progresses. Eventually, hemodialysis (HD) or peritoneal dialysis (PD) must be initiated to replace kidney function in most patients. Approximately 100,000 new patients began receiving HD in the United States in 2006; over 325,000 patients received hemodialysis treatments that year. These numbers accounted for approximately 92% of the total dialysis population. Patients on PD accounted for 6.2% of new dialysis cases, and 8.2% of the total dialysis cases ( NIDDKD, 2008 ).
The debate continues over which treatment modality is more effective and has better outcomes. No conclusive evidence indicates one form of dialysis is better than the other ( Lee, Sun, & Wu, 2009 ). Many factors must be considered when choosing a dialysis modality, including physician recommendation, patient preference, and availability of the treatment ( Shahab, Khanna, & Nolph, 2006 ). Dialysis modality selection often is based on the patient’s clinical and social status ( Shahab et al., 2006 ). In general, patients who are younger and adherent to therapy; have some residual renal function, cardiovascular disease, and family or social support; and are independent in self-care are more likely to be recommended for peritoneal dialysis. Peritoneal dialysis is contraindicated in patients who have ostomies, or a ventro-peritoneal shunt. Older adults, obese persons, and individuals without social support also are less likely to be recommended for peritoneal dialysis ( Shahab et al., 2006 ).
Fatigue in ESRD
The need to identify and assess fatigue in patients receiving dialysis is vital to patient health and quality outcomes. Fatigue frequently is unrecognized and therefore under-treated ( Jhamb, Weisbord, Steel, & Unruh, 2008 ). Physical exercise, epoetin use, and L-carnitine infusion have all been used successfully to alleviate fatigue in patients receiving hemodialysis. Physical exercise also can help with the physiological and functional deterioration that can result from aging, illness, and sedentary lifestyle ( Gordon, Doyle, & Johansen, 2011 ; Kosmadakis et al., 2010 ), all of which can contribute to dialysis-related fatigue.
Trials have shown physical exercise is safe for patients on dialysis, but exercise is not offered or recommended routinely for patients receiving dialysis. While physical exercise may be safe and help alleviate fatigue, few patients participate in these programs when offered, and many often drop out after beginning ( Kosmadakis et al., 2010 ). Additionally, exercise may not be appropriate for a select group of patients receiving dialysis. Patients with functional limitations, poor cardiac health, or bone disease, and persons who are hemodynamically unstable during dialysis should not be considered for participation in exercise programs ( Bayliss, 2006 ).
Epoetin is used regularly to combat anemia, a common cause of dialysis-related fatigue. Some patients do not respond well to epoetin therapy ( Bamgbola, 2011 ) as demonstrated by no increase in their hemoglobin and hematocrit levels. Unfortunately, increasing doses of epoetin to help reduce anemia in these patients has proven detrimental, as it increases their risk for cardiovascular and cerebrovascular events ( Drueke et al., 2006 ; Singh et al., 2006 ). Therefore, epoetin use may not be successful in alleviating fatigue in all patients receiving hemodialysis.
Another intervention to lessen the effects of fatigue is the use of L-carnitine, which is important for appropriate muscle function. Patients receiving hemodialysis are deficient in L-carnitine, and supplementation has proven effective in ameliorating dialysis-related fatigue, particularly in patients who are unresponsive to epoetin therapy ( Lynch et al., 2008 ). The Centers for Medicare and Medicaid Services (CMS) only reimburses for L-carnitine used for epoetin-resistant anemia and intradialytic hypotension. Continued use of the drug is not covered if there has been no improvement in anemia or hypotension 6 months after treatment initiation ( CMS, 2011 ).
Interventions that have been successful in alleviating fatigue may not be appropriate or safe for all patients. A significant need exists for the management of fatigue in order to reduce its impact on the lives of patients receiving hemodialysis. Nurses are in a strategic position to assess dialysis-related fatigue and help patients develop strategies to manage its effects. The following case study describes a patient admitted to the medical-surgical unit for management of hyperglycemia. The assigned nurse meets the patient for the first time immediately after her dialysis session. Patient symptoms, assessment of fatigue, evaluation of patient medications and lab results, and nursing interventions are discussed.
Mrs. R. is a 33-year-old African-American female with type 1 diabetes, cataracts, and ESRD who has received hemodialysis for 2 years. She was admitted in the morning for hyperglycemia and management of medications, and was taken to dialysis prior to her arrival on the medical-surgical unit. After arrival to her room, she is lethargic, replies to repeated questions slowly, and slurs words at times. She demonstrates delayed response to commands with appropriate one-word answers. When asked how she feels, she responds, “I’m so tired. Please just leave me alone.” Dialysis removed 5 kg of fluid. Vital signs include oral temperature 98.3° F, blood pressure 88/40 mm Hg, pulse 96 beats per minute, respirations 12 breaths per minute, and oxygen saturation 97% on room air. See Table 2 for the patient’s routine medications, and Table 3 for current laboratory results. The patient’s height is 5′5″ and weight 145 lbs. Mrs. R.’s history includes childhood non-adherence to diabetic diet and medications, which contributed to her current health status. She lives with her mother, receives disability payments and is unemployed, and has a 9-year-old daughter. She is unable to drive due to poor vision.
Current Medications
| Name | Dosage | Times |
|---|---|---|
| Insulin glargine (Lantus insulin) | 28 units | Q a.m. and p.m. |
| Regular insulin (Humulin R ) | Sliding scale | QID |
| Atenolol (Tenormin ) | 50 mg | BID |
| Clonidine (Catapres ) | 0.1 mg | BID |
| Calcium acetate (PhosLo ) | 3 tabs | With meals |
| Epoetin alfa (Epogen ) | 100 u/kg | TIW |
| Ferrous sulfate | 325 mg | BID |
| Alprazolam (Xanax ) | 0.25 mg | BID PRN |
| Docusate sodium (Colace ) | 2 tabs | QD |
| Calcitriol (Rocaltrol ) | 0.25 mg | QD |
| Atorvastatin (Lipitor ) | 20 mg | QD |
| Venlafaxine (Effexor ) | 150 mg | QD |
Current Lab Values
| Lab Test | Value |
|---|---|
| K | 3.4 mEq/L |
| Na | 136 mEq/L |
| Ca | 9.3 mg/dL |
| PO | 2.8 mg/dL |
| Mg | 1.5 mEq/L |
| Total protein | 5.5 g/dL |
| Albumin | 3.3 g/dL |
| Hgb | 10 g/dL |
| Hct | 25% |
| Glucose | 288 |
Nursing Management
Fatigue assessment.
Mrs. R. presents as a typical patient with ESRD. Her fatigue is the result of both physiological and psychological influences as well as dialysis treatment inadequacy. When questioned further about her fatigue, the patient explains, “It’s ok; I’m always like this after dialysis.” Emergency interventions are not needed, but assessment of the fatigue should be completed. A simple visual analogue scale could be used to establish Mrs. R.’s baseline perception of fatigue; the scale includes a 100 mm line anchored at the left end with No Tiredness and at the right end with Complete Exhaustion . This type of measurement is a reliable assessment of fatigue in patients receiving dialysis ( Brunier & Graydon, 1996 ; Williams et al., 2007 ), and can be performed quickly in the clinical setting.
Evaluate How Fatigue Impacts Patient’s Daily Living
Because fatigue can be extremely debilitating, assessing its effects on Mrs. R. is important in helping her improve her quality of life. Assessment can include asking the patient to describe her daily routine or keep a journal for review with the nurse at the dialysis unit at a later date. Mrs. R. explains that she sleeps for 4–6 hours after dialysis because she is completely exhausted. She is unable to work due to her extreme dialysis-related fatigue, and therefore receives disability payments. She lives with her mother to save rent money and receive help from her mother in caring for her 9-year-old daughter. Reviewing a time or event when fatigue was at its greatest intensity may help with planning interventions to minimize fatigue. Mrs. R. states that at times, particularly on dialysis days, she is unable to fix her daughter meals or help with her homework. The nurse can help Mrs. R. identify her support systems and collaborate with her to identify ways she can incorporate these support systems into her life. For instance, could Mrs. R.’s mother help her granddaughter with homework after school and cook evening meals? If Mrs. R. is able, making meals on her non-dialysis days and refrigerating or freezing them will help her mother and make food available when she is unable to prepare it.
Evaluating Laboratory Results and Medications
Irregularities in laboratory results and medication side effects may contribute to Mrs. R.’s fatigue. Evaluation of the patient’s laboratory results reveals serum sodium, calcium, phosphorus, and magnesium are all within normal range albeit slightly low. Her slight hypokalemia could be related to the stress of dialysis. Dialysis can return many electrolyte values to normal ranges, but also may remove insulin. Her serum glucose is elevated, which can lead to cell dehydration due to osmotic pressure in the extracellular fluid. She may need regular insulin for glucose correction. The presence of insulin affects protein (amino acids), glucose, potassium, magnesium, and phosphate uptake by the cells. Without insulin, the cells do not have the adequate glucose and amino acids to function, and weakness and/or fatigue can result. Mrs. R.’s protein and albumin values are already low, which also can cause edema. This in turn can lead to swelling and weight gain, causing tightly fitting clothes and shoes that can affect movement and increase feelings of fatigue. Dialysis removed 5 kg of fluid, which most likely included toxic levels of electrolytes, a small amount of blood, and other waste products. Mrs. R.’s hemoglobin and hematocrit are low, often typical for a patient in ESRD. With low protein and albumin, she also may lack elements to produce red blood cells. The kidney’s ability to make erythropoietin, which stimulates red blood cell production, is compromised and could contribute to Mrs. R.’s fatigue. Epoetin has been prescribed to supplement her kidneys’ failing ability to produce erythropoietin. Supplemental iron and vitamin B 12 also may be given. By increasing hemoglobin, patients may experience less lethargy.
Mrs. R. also takes alprazolam (Xanax ® ) twice a day as needed for restless leg syndrome. This drug also may be contributing to daytime sleepiness and fatigue. The nurse should encourage Mrs. R. to investigate medications other than benzodiazapines for restless leg syndrome with her care provider to alleviate some of the fatigue. Mrs. R. also takes the anti-depressant venlafaxine (Effexor ® ). Depression has been associated with dialysis-related fatigue in several studies ( Kim & Son, 2005 ; Leinau et al., 2009 ; Liu, 2006 ; McCann & Boore, 2000 ), and it is important that depression is identified and treated. Treating depression could help lessen fatigue levels in patients receiving dialysis. In Mrs. R.’s case, the nurse should ask if she believes her current medication is treating her depression effectively. If depression is under-treated, her fatigue may persist.
Teaching the Patient
After examining the patient’s laboratory results and medications, the nurse should teach the patient the importance of diet, exercise, and healthy sleep routines to decrease symptoms of fatigue. Diet is critically important for patients receiving dialysis. Mrs. R.’s diet should be low in potassium, sodium, and phosphate, and her fluid intake restricted. She needs adequate calories as well as moderate intake of complete protein, low fat, vitamins A and C, and carotinoids. Over time, patients affected with ESRD tend to become malnourished, so aggressively restricting their protein intake may be more detrimental to their health ( Arora & Verrell, 2009 ). Because Mrs. R. also has diabetes, she needs high-complex, fiber-rich carbohydrates with low glycemic index and load necessary for calorie intake ( Taillefer, 2008 ). Collaborating with a registered dietitian and designing menus for Mrs. R. may decrease the burden of planning meals.
Exercise is another essential component of patient teaching. Mrs. R. has none of the previously mentioned complications that would prevent her from participating in exercise. Collaborating with her in planning simple exercises is a must. Suggesting an exercise routine during the dialysis appointment can encourage adherence as well as socialization with others. Thirty minutes of low-intensity exercise, such as modified yoga, should be the goal ( Yurtkuran, Alp, Yurtkuran, & Dilek, 2007 ). Some exercise plans include 30 minutes of cycling with devices adapted to the patient’s bed during dialysis ( Quzouni, Kouidi, Sioulis, Grekas, & Deligiannis, 2009 ). Exercise can lead to significant improvements in the patient’s physical abilities, as well as decreased perception of pain, fatigue, depression, and insomnia.
Sleep disorders, such as apnea and restless leg syndrome, are common among patients receiving dialysis. In addition to management with medication, the patient may need to make behavioral changes, such as staying awake during dialysis and eliminating caffeine, nicotine, and alcohol intake. Some units provide overnight dialysis, which might be helpful. Sleep studies should be performed for sleep apnea and restless leg syndrome with the appropriate interventions of continuous positive airway pressure usage or medications ( Unruh, 2008 ).
Evaluating this patient’s sleep and her participation in routine exercise can improve feelings of depression as well. Her loss of self, a change of status in her family, the inability to care for her daughter, her physical losses, and a loss of a social network can contribute to depression. The nurse should help the patient identify support people and local support groups, listing ways she has coped with crisis in the past. The nurse also can help her establish goals to give her a sense of hope. She may need a professional therapist, guidance in time management, and a social worker to find resources for caring for herself and daughter.
Fatigue is a real problem for patients receiving dialysis. While the specific cause of fatigue remains unknown, multiple conditions are associated with its occurrence. Nursing assessment of fatigue is important in the care of patients receiving dialysis in order to improve their quality of life. Nurses are in an excellent position to review patients’ medications and laboratory results, and collaborate with patients to determine how to use their support systems and individual strengths to help alleviate the effects of fatigue.
Acknowledgments
This project was supported by a grant from the National Center for Research Resources, Duke CTSA, NIH, grant number 1TL1RR024126. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Contributor Information
Ann Horigan, Duke University School of Nursing, Harrisonburg, VA.
Judith Rocchiccioli, James Madison University, Harrisonburg, VA.
Donna Trimm, James Madison University, Harrisonburg, VA.
- Alper AB, Shenava RG, Young BA. Uremia. 2010 Retrieved from http://emedicine.medscape.com/article/245296-overview .
- Arora P, Verrell M. Chronic kidney disease treatment & management. 2009 Retrieved from http://emedicine.medscape.com/article/238798-treatment .
- Bakris GL. Recognition, pathogenesis and treatment of different stages of nephropathy in patients with Type 2 diabetes mellitus. Mayo Clinic Proceedings. 2011; 86 (5):444–456. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bamgbola OF. Pattern of resistance to erythropoietin-stimulating agents in chronic kidney disease. Kidney International. 2011; 80 (5):464–474. doi: 10.1038/ki.2011.179. [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bayliss D. Starting and managing an intradialytic exercise program. Nephrology News and Issues. 2006; 20 (9):47–49. [ PubMed ] [ Google Scholar ]
- Broscious SK, Castagnola J. Chronic kidney disease: Acute manifestations and role of critical care nurses. Critical Care Nurse. 2006; 26 (4):17–20. 22–27. [ PubMed ] [ Google Scholar ]
- Brunier G, Graydon J. A comparison of two methods of measuring fatigue in patients on chronic hemodialysis: Visual analogue vs Likert scale. International Journal of Nursing Studies. 1996; 33 (3):338–348. [ PubMed ] [ Google Scholar ]
- Castner D. Understanding the stages of chronic kidney disease. Nursing. 2010; 40 (5):24–31. [ PubMed ] [ Google Scholar ]
- Centers for Medicare and Medicaid Services (CMS) Levocarnitine for use in the treatment of carnitine deficiency in ESRD patients. Medicare National Coverage Determinations Manual. 2011 section 230.19 Retrieved from http://www.cms.gov/manuals/downloads/ncd103c1_Part4.pdf .
- Drueke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Scherhag A. Normalization of hemoglobin level in patients with CKD and anemia. New England Journal of Medicine. 2006; 355 :2084–2006. [ PubMed ] [ Google Scholar ]
- Gordon PL, Doyle JW, Johansen KL. Postdialysis fatigue is associated with sedentary behavior. Clinical Nephrology. 2011; 75 (5):426–433. [ PubMed ] [ Google Scholar ]
- Hill GS. Hypertensive neprhrosclerosis. Current Opinion in Nephrology and Hypertension. 2008; 17 (3):266–270. [ PubMed ] [ Google Scholar ]
- Jablonski A. The multidimensional characteristics of symptoms reported by patients on hemodialysis. Nephrology Nursing Journal. 2007; 34 (1):29–37. [ PubMed ] [ Google Scholar ]
- Jhamb M, Weisbord S, Steel J, Unruh M. Fatigue in patients receiving maintenance dialysis: A review of definitions, measures, and contributing factors. American Journal of Kidney Disease. 2008; 52 (2):353–365. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kim H, Son G. Fatigue and its related factors in Korean patients on hemodialysis. Taehan Kanho Hakhoe Chi. 2005; 35 (4):701–708. [ PubMed ] [ Google Scholar ]
- Kinzner C, Hain D. Understanding the eGFR. Nephrology Nursing Journal. 2007; 34 (6):655–657. [ PubMed ] [ Google Scholar ]
- Kosmadakis GC, Bevington A, Smith AC, Clapp EL, Viana JL, Bishop NC, Feehally J. Physical exercise in patients with severe kidney disease. Nephron Clinical Practice. 2010; 115 (1):c7–c16. [ PubMed ] [ Google Scholar ]
- Lee CC, Sun CY, Wu MS. Long-term modality related-mortality analysis in incident dialysis patients. Peritoneal Dialysis International. 2009; 29 (2):182–190. [ PubMed ] [ Google Scholar ]
- Leinau L, Murphy T, Bradley E, Fried T. Relationship between conditions addressed by hemodialysis guidelines and non-ESRD-specific conditions affecting quality of life. Clinical Journal of the American Society of Nephrology. 2009; 4 (3):572–578. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Liu H. Fatigue and associated factors in hemodialysis patients in Taiwan. Research in Nursing and Health. 2006; 29 (1):40–50. [ PubMed ] [ Google Scholar ]
- Lynch KE, Feldman HI, Berlin JA, Flory J, Rowan CG, Brunelli SM. Effects of L-carnitine on dialysis related hypotension and muscle cramps: A meta-analysis. American Journal of Kidney Diseases. 2008; 52 (5):962–1071. [ PubMed ] [ Google Scholar ]
- McCance K, Huether S. Pathophysiology: The biologic basis for disease in adults & children. 6. St. Louis, MO: Mosby; 2010. [ Google Scholar ]
- McCann K, Boore J. Fatigue in persons with renal failure who require maintenance haemodialysis. Journal of Advanced Nursing. 2000; 32 (5):1132–1142. [ PubMed ] [ Google Scholar ]
- McCarley PB, Arjomand M. Mineral and bone disorders in patients on dialysis: Physiology and clinical consequences. Nephrology Nursing Journal. 2008; 35 (1):59–64. [ PubMed ] [ Google Scholar ]
- Molzahn AE, Butera E, editors. Contemporary nephrology nursing: Principles and practice. 2. Pitman, NJ: American Nephrology Nurses Association; 2006. [ Google Scholar ]
- Murphy F, Jenkins K, Chamney M, McCann M, Sedgewick J. Patient management in CKD stages 1 to 3. Journal of Renal Care. 2008; 34 (3):127–135. [ PubMed ] [ Google Scholar ]
- Murtaugh F, Addington-Hall J, Higginson I. The prevalence of symptoms in end stage renal disease: A systematic review. Advances in Chronic Kidney Disease. 2007; 14 (1):82–99. [ PubMed ] [ Google Scholar ]
- National Institute of Diabetes and Digestive and Kidney Disease (NIDDKD) United States Renal Data System 2010 annual data report: Atlas of chronic kidney disease and end stage renal disease in the United States. Bethesda, MD: National Institutes of Health; 2010. [ Google Scholar ]
- National Institute of Diabetes and Digestive and Kidney Diseases (NIDDKD) Chronic kidney disease-mineral bone disorder. 2009a Retrieved from http://kidney.niddk.nih.gov/kudiseases/pubs/CKD_Mineral_Bone/index.htm .
- National Institute of Diabetes and Digestive and Kidney Diseases (NIDDKD) The kidneys and how they work. 2009b Retrieved from http://kidney.niddk.nih.gov/kudiseases/pubs/yourkidneys/yourkidneys_508.pdf .
- National Institute of Diabetes and Digestive and Kidney Disease (NIDDKD) United States Renal Data System 2008 annual data report: Atlas of chronic kidney disease and end stage renal disease in the United States. Bethesda, MD: National Institutes of Health; 2008. [ Google Scholar ]
- National Kidney Foundation (NKF) K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. American Journal of Kidney Diseases. 2002; 39 (2):S46–S75. [ PubMed ] [ Google Scholar ]
- National Kidney Foundation (NKF) About chronic kidney disease. 2011 Retrieved from http://www.kidney.org/kidneydisease/ckd/index.cfm#whatare .
- O’Sullivan D, McCarthy G. An exploration of the relationship between fatigue and physical functioning in patients with end stage renal disease receiving haemodialysis. Journal of Clinical Nursing. 2007; 16 (11C):276–284. [ PubMed ] [ Google Scholar ]
- Putcha N, Allon M. Management of hyperkalemia in dialysis patients. Seminars in Dialysis. 2007; 20 (5):431–439. [ PubMed ] [ Google Scholar ]
- Quzouni S, Kouidi E, Sioulis A, Grekas D, Deligiannis A. Effects of intra-dialytic exercise training on health-related quality of life indices in haemodialysis patients. Clinical Rehabilitation. 2009; 23 (1):53–63. [ PubMed ] [ Google Scholar ]
- Redmond A, McClelland H. Chronic kidney disease: Risk factors, assessment and nursing care. Nursing Standard. 2006; 20 (10):48–55. [ PubMed ] [ Google Scholar ]
- Shahab I, Khanna R, Nolph K. Peritoneal dialysis or hemodialysis? A dilemma for the nephrologist. Advances in Peritoneal Dialysis. 2006; 22 :180–185. [ PubMed ] [ Google Scholar ]
- Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D, CHOIR Investigators. Correction of anemia with epoetin alpha in CKD. New England Journal of Medicine. 2006; 355 :2085–2098. [ PubMed ] [ Google Scholar ]
- Taillefer TL. Nurses and dietitians collaborating to impact nutrition and diabetes mellitus management issues for patients with type 2 diabetes mellitus on hemodialysis. Nephrology Nursing Journal. 2008; 35 (5):503–505. [ PubMed ] [ Google Scholar ]
- Unruh M. Sleep disorders in chronic kidney disease. Primary Psychiatry. 2008; 15 (1):57–63. [ Google Scholar ]
- Weisbord S, Fried L, Arnold R, Fine M, Levenson D, Peterson R, Switzer G. Prevalence, severity, and importance of physical and emotional symptoms in chronic hemodialysis patients. Journal of the American Society of Nephrology. 2005; 16 (8):2487–2494. [ PubMed ] [ Google Scholar ]
- Williams A, Crane P, Kring D. Fatigue in African American women on hemodialysis. Nephrology Nursing Journal. 2007; 34 (6):610–617. [ PubMed ] [ Google Scholar ]
- Yurtkuran M, Alp A, Yurtkuran M, Dilek K. A modified yoga-based exercise program in hemodialysis patients: A randomized controlled study. Complementary Therapies in Medicine. 2007; 15 (3):164–171. [ PubMed ] [ Google Scholar ]
Population Health
How Nurse Staffing Variations were Associated with Hospital Patient Deaths During the COVID Pandemic
New study looks back at conditions that speak to future hospital emergency preparedness.
- Hoag Levins
- Share this page on Twitter
- Share this page on Facebook
- Share this page on LinkedIn
The only study to evaluate the association of hospital nurse staffing level variations and patients’ odds of dying during the COVID-19 emergency concluded that many deaths among patients hospitalized for COVID-19 could have been prevented if hospitals entered the pandemic with adequate numbers of registered nurses (RNs), a workforce rich in Bachelor of Science degree qualified RNs, and high-quality nurse work environments.
Titled “ Hospital Nursing Staffing Variation and COVID-19 Deaths: A Cross-Sectional Study ,” the paper was co-authored by LDI Senior Fellows and University of Pennsylvania School of Nursing faculty members Karen B. Lasater, PhD, RN ; Matthew D. McHugh, PhD, RN ; and Linda H. Aiken, PhD, RN .

Published in the International Journal of Nursing Studies , the work adds to a large body of evidence generally associating nurse staffing variations to trends in patient outcomes and deaths at the same time it underscores elements of the national debate about the overall adequacy of hospital emergency preparedness during the pandemic as well as for the next major national health emergency.
No Meaningful Improvement
“Many hospitals were chronically understaffed prior to the pandemic, and on average, we found that staffing levels did not meaningfully improve during the pandemic to meet the accelerating care needs of very sick and medically complex patients,” said lead author Lasater.
The study pointed out that during the pandemic, COVID-19 mortality varied depending on the hospital where patients were admitted, but it was unknown what aspects of the hospitals’ operations were important for mitigating preventable deaths.
The study objective was to “determine whether hospital differences in pre-pandemic and during pandemic nursing resources—average patient-to-registered nurse (RN) staffing ratios, proportion of bachelor-qualified RNs, nurse work environments, Magnet recognition—explain differences in risk-adjusted COVID-19 mortality; and to estimate how many deaths may have been prevented if nurses were better resourced prior to and during the pandemic.”
The term “Magnet recognition” refers to certification by the American Nurses Association’s (ANA) American Nurses Credentialing Center (ANCC) that a hospital follows an evidence-based standard for transformational nursing leadership, empowerment of front-line nurses, evidenced-based professional nursing practice, and high-quality care, known to minimize clinician stress and improve patient care.
Thousands of Preventable Deaths
The investigation evaluated data from 237 hospitals and more than 87,000 older adult patients admitted with COVID-19 during the height of the pandemic and found that “variation in hospital nursing resources prior to the COVID-19 pandemic is associated with many thousands of preventable COVID-19 deaths during the pandemic.”
One study finding that directly relates to the outlook for current and future hospital emergency preparedness was that “Hospital nursing resources are often overlooked in conversations about lessons from the U.S. COVID-19 response and hospitals have not yet been able to recover from the effects of COVID-19 as they continue to experience difficulties recruiting and retaining nurses after the official end of the pandemic.”
And as further context for the issue, the study points back to multiple previous works that looked at nursing during the pandemic and found “serious misalignments” between the resources nurses were provided with and what they actually needed to optimize patient care. One of those earlier studies found that the most important resources nurses said they needed in order to provide safe and quality care during and after the COVID-19 emergency were flexible work scheduling and adequate staffing. But more than two-thirds of nurses surveyed reported these resources were not provided to them by their hospitals.
A currently related controversy suggests that insufficient RN staffing is associated with the lack of sufficient registered nurses available in the U.S. But Lasater pointed to a just-released national analysis by Marsh McLennan’s Mercer, a human resources and financial services consulting firm. It reports that, “At a national level, the supply of RNs is projected to outpace demand, resulting in an estimated surplus of nearly 30,000 RNs by 2028.”
Nurses Flee Poor Working Conditions
In previous papers, Lasater has explained that the current insufficient numbers of nurses in hospitals is not a function of overall supply but rather poor working conditions in hospitals that drive high clinician burnout, job dissatisfaction, turnover, and poor patient outcomes. Other recent evidence documents the continued growth in the number of newly licensed nurses but notes a shift in employment away from hospital settings toward ambulatory and community settings.
The American Nurses Association (ANA) endorses safe staffing ratios that would require hospitals to meet a minimum standard level of nursing care. Meanwhile, California and Oregon have already established enforceable staffing ratio requirements. As a result, patients hospitalized in California receive an average of three more hours of RN care per day compared to patients in other states. Oregon implemented their ratio policy in the summer of 2024.
Other U.S. jurisdictions including Pennsylvania, Maine, Georgia, Illinois, and New Jersey have all introduced safe staffing bills. Elsewhere in the world, a prospective evaluation of staffing ratios in Queensland Australia showed the introduction of staffing legislation in hospitals resulted in better staffing ratios, lower mortality, fewer readmissions, and shorter stay durations for patients.
A National Priority
When asked what policymakers can take away from her new study findings, Lasater said, “Federal and state policies that address the underlying issue of chronic nurse understaffing requires action today. If having enough nurses to care for the public and prevent avoidable deaths during future public health emergencies is a national priority, action is needed to prevent chronic understaffing of nurses during normal times.”
More LDI News

Health Equity
How Low Trust in Government May Hurt Clinical Trial Participation
Evidence from Philadelphia During the Pandemic Suggests Ways To Build Trust
- Miles Meline, MBE
- and Mackenzie Bolas
Health Care Access & Coverage
What Happens When a Multihospital System Buys an Independent Hospital?
System Acquisitions Increase Prices, Reduce Staffing, and May Affect Quality
- Chris Tachibana, PhD, MS

Sustainable Funding and Equitable Access for Multimillion Dollar Gene Therapies
Penn LDI Panel Unpacks Barriers and Options for Change
Medicaid Could Become a Powerful Tool to Improve Racial and Ethnic Representation in Cancer Clinical Trials
One Key May Be Medicaid’s Recent Coverage of the Routine Costs for Enrolling in Clinical Trials
- Paula Chatterjee, MD, MPH

Hospital Settings Can Be a Form of Treatment by Themselves
Patients Need Nature Enriched Rooms With Access to Peaceful, Outdoor Settings
- Meghan Crnic, PhD and Dominic A. Sisti, PhD, MBE
This Parent Screening Tool Seeks to Normalize Gender Conversations by Measuring Families’ Alignment and Distress
Pioneering Tool Aims to Integrate Gender Discussions in Pediatric Care
- Mackenzie Bolas

IMAGES
VIDEO
COMMENTS
In contrast, patients receiving hemodialysis more often experienced worse quality of sleep, chronic fatigue, pain, and vomiting. Similar results were obtained by Turkmen et al 20 and Theofilou 21 who showed that in a group of patients receiving hemodialysis 41%-83% experienced sleep disorder and chronic weakness.
2 - 4. Twelve articles described good practices for dialysis education (Table 2), three for dialysis treatment (Table 3), and four for eHealth (Table 4). All articles were published during the past 20 years and 47% of them came from the United States of America (USA). Most studies (58%) had a qualitative design, while the others were cohort ...
Introduction. People with end-stage kidney disease require the life-sustaining therapy of dialysis or a renal transplant. The most prevalent form of dialysis in the United States and Canada is in-center hemodialysis (HD) requiring the person to travel to the dialysis center 3 times a week ().People on HD have decreased levels of physical functioning which is predictive of poor patient outcomes ().
Background Recommendations regarding dialysis education and treatment are provided in various (inter)national guidelines, which should ensure that these are applied uniformly in nephrology and dialysis centers. However, there is much practice variation which could be explained by good practices: practices developed by local health care professionals, which are not evidence-based. Because an ...
Journal of Renal Care is an international, peer-reviewed nephrology journal for researchers and healthcare professionals caring for people with kidney disease. ... The European Dialysis and Transplant Nurses Association/European Renal Care Association. More from this journal News; Reviewers 2018; Patient Editor's Choice; Virtual Issue ...
Meeting the Emotional Needs of Hemodialysis Patients and Their Spouses. SANTOPIETRO, MARY-CHARLES S. AJN, American Journal of Nursing. 75 (4):629-632, April 1975. Through a study of home- hemodialysis patients and their wives, the author identifies six areas in which nurses can help these couples. Abstract.
In a 2017 survey of 125 countries, PD was reportedly available in 75% of countries whereas haemodialysis was available in 96% 20. In 2018, an estimated 11% of patients receiving long-term dialysis ...
The good practices for dialysis treatment focus mainly on dialysis access devices and general quality improvement of dialysis care. Finally, eHealth is useful for HD and PD and affects both quality of care and health-related quality of life. Conclusion: Our scoping review identifies 19 articles describing good practices and their results for ...
This article is part of a series, Supporting Family Caregivers: No Longer Home Alone, published in c ... American Journal of Nursing: January 2021 - Volume 121 - Issue 1 - p 57-63. doi: 10.1097/01.NAJ.0000731684.93883.38 ... Peritoneal dialysis patients and their caregivers should notify the dialysis provider any time they believe they may have ...
The Journal of Clinical Nursing publishes research and developments relevant to all areas of nursing practice- community, geriatric, mental health, pediatric & more. Abstract Aim To identify and synthesise qualitative studies on barriers and facilitators perceived by dialysis patients in relation to self-care and disease management.
Affiliations. 1 Acute Dialysis Nurse, DCH Regional Medical Center, Tuscaloosa, AL. 2 American Nephrology Nurses Association (ANNA) National Director (2019-2021). 3 member of ANNA's Hazel Taylor Chapter. 4 Director, Community Health Initiatives, and Director of Dialysis/Apheresis, Peninsula Regional Medical Center, Delmar, DE.
In the United States, 34% of hospital discharges among patients receiving dialysis are followed by a 30-day readmission,1 and both dialysis clinics2 and hospitals3 are held accountable for readmissions (through potential payment reductions). Although improved care coordination between hospitals and dialysis clinics may play an important role in reducing hospital readmissions and improving ...
People with kidney failure undergoing dialysis frequently report distressful symptoms, such as fatigue, pain, and pruritus. These symptoms have negative impacts on quality of life and other clinical outcomes, including health care utilization and mortality risk. Despite these tremendous impacts, symptom assessment and management can be challenging in clinical settings.1 In May 2022, Kidney ...
Published November 4, 2010. N Engl J Med 2010;363: 1833 - 1845. DOI: 10.1056/NEJMra0902710. VOL. 363 NO. 19. Fifty years ago, Belding Scribner and his colleagues at the University of Washington ...
In the United States, increasing numbers of elderly patients with end-stage renal disease (ESRD) are starting dialysis. 1 In 1999, nursing home residents accounted for 4% of all new patients with ...
The Nephrology Nursing Journal is designed to meet the educational and information needs of nephrology nurses in a variety of roles at all levels of practice. It also serves as a source for nonnephrology nurses. Its content expands the knowledge base for nephrology nurses, stimulates professional growth, guides research-based practice, presents new technological developments, and provides a ...
Nephrology nursing journal: journal of the American Nephrology Nurses' Association. 2012;39(3):217-28. ... Chen W, Lu XH, Wang T. Menu suggestion: an effective way to improve dietary compliance in peritoneal dialysis patients. Journal of renal nutrition: the official journal of the Council on Renal Nutrition of the National Kidney Foundation ...
For over a decade, nephrology nurses, including those with dialysis expertise, have been in short supply, reflecting a broader nursing shortage in the United States.1,2 By 2030, experts project a national deficit of more than 900,000 nurses, largely attributed to a growing elderly population and limitations in nursing school capacity.3,4 Vacancies across nursing positions are estimated at 8% ...
Coping with chronic kidney disease (CKD) is challenging for many people, since symptoms often don't appear until the disease is advanced and the patient is close to requiring dialysis. This two-part article aims to provide nurses with the basic information necessary to assess and manage patients with CKD. Part 1, which appeared last month, offered an overview of the disease, described ...
1. Introduction. Chronic renal failure [] is secondary to the continuous progression of various chronic kidney diseases and is characterized by renal insufficiency, metabolite retention, dysregulation of the internal environment, and functional imbalance of systems.Clinical statistics show that the annual incidence of chronic renal failure is about 0.3%, which exhibits an increasing trend in ...
Read papers from Nephrology Nursing Journal : Journal of the American Nephrology Nurses' Association with Read by QxMD. ... as a clinical practice project to re-evaluate how training and education are provided to individuals working in the dialysis setting. This article describes an education initiative based on the escape room methodology to ...
By 2030, an estimated 5.4 million people globally will be receiving kidney replacement therapy (KRT) for the treatment of kidney failure. 1 Although increasing numbers of adults 65 years and older are initiating KRT, there is a growing body of literature suggesting that a subset of patients, particularly those older than 75 years who have serious illnesses such as dementia or ischemic heart ...
BACKGROUND: Fatigue is a pervasive and debilitating symptom among hemodialysis patients, severely impacting their quality of life and ability to participate in social activities. Dialysis nurses are pivotal in alleviating these effects through physical exercise. This study aims to evaluate the effectiveness of an integrated dialysis nursing intervention in reducing fatigue among hemodialysis ...
Fatigue is one of the most common symptoms experienced by patients receiving dialysis. When patients with chronic kidney disease (CKD) and end-stage renal disease are admitted to acute care settings, they require management of their often profound fatigue. CKD, renal pathology, and renal fatigue are examined in relation to a case study. Caring ...
Published in the International Journal of Nursing Studies, the work adds to a large body of evidence generally associating nurse staffing variations to trends in patient outcomes and deaths at the same time it underscores elements of the national debate about the overall adequacy of hospital emergency preparedness during the pandemic as well as for the next major national health emergency.